UCL Professor of Cell Biology
+44 (0)20 7679 7808
LMCB Room B1.05
Endothelial cell biology
Research synopsis
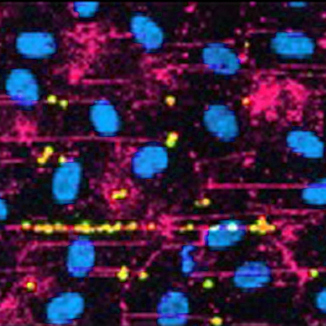
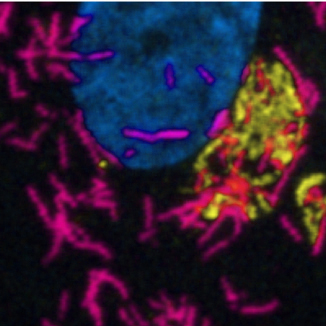
The endothelial cells that line the vasculature contain a storage organelle, Weibel-Palade Bodies (WPB). The major content protein of WPB is von Willebrand factor (VWF), an adhesive glycoprotein that plays a major role in primary haemostasis. As a rapid-response system, the WPB are thus of critical importance in initiating haemostatic responses within the rapidly-changing vascular environment.
Mutations within VWF are the commonest cause of the inherited bleeding disorder Von Willebrands Disease. In addition to mutations causing a loss of platelet binding or other direct haemostatic problems, mutations in VWF can affect the formation of the protein tubules, leading to formation of damaged, poorly functional WPB.
Advances in microscopy and in bioinformatics revealed that WPB size increases in half-micron steps. VWF subunits, "quanta" are brought together in the trans-Golgi where they assemble into organelles with a 10-fold range in length. By manipulating WPB length, we have shown that short WPB are much less efficient at supporting platelet recruitment, and thus initiating primary haemostasis.
We then went on to discover how the cell controls the size of WPB in response to metabolic changes, thereby controlling the haemostatic response to physiological circumstances.
We are now exploring the effect of using human licensed drugs identified by screens to shrink WPB, as a way to counteract excess haemostasis in models of disease.
Selected publications
Patella F, et al (2021). Shrinking Weibel-Palade bodies prevents high platelet recruitment in assays using thrombotic thrombocytopenic purpura plasma. Res Pract Thromb Haemost. 2021;5: e12626
Ferraro F, et al. (2020). Modulation of endothelial organelle size as an antithrombotic strategy. J Thromb. Haemost. 18:3296-3308
Lopes da Silva M, et al (2019). A GBF1-Dependent Mechanism for Environmentally Responsive Regulation of ER-Golgi Transport. Dev Cell. 2019 Jun 3;49(5):786-801.e6. doi: 10.1016/j.devcel.2019.04.006.
Nightingale TD, et al (2018). Tuning the endothelial response: differential release of exocytic cargos from Weibel-Palade bodies. J Thromb Haemost. 2018 Sep;16(9):1873-1886. doi: 10.1111/jth.14218.
McCormack JJ, et al (2017). Weibel-Palade bodies at a glance. J Cell Sci. 2017 Nov 1;130(21):3611-3617. doi: 10.1242/jcs.208033.
Lopes da Silva M & Cutler DF (2016). Von Willebrand Factor multimerization and the polarity of secretory pathways in endothelial cells. Blood.
Ferraro F, et al (2014). A two-tier Golgi-based control of organelle size underpins the functional plasticity of endothelial cells. Dev Cell, 29 (3), 292-304. doi:10.1016/j.devcel.2014.03.021
About the lab
Funders
Medical Research CouncilBritish Heart Foundation
Research themes
Polarity and cell shape
Cytoskeleton and cell cortex
Organelle biogenesis
Endothelial cells
Platelets
Haemostasis
Membrane trafficking
Inflammation
Technology
Light microscopy
Translational research
Bioinformatics
Super-resolution microscopy
Electron microscopy
People
Collaborators
Louise Cramer (LMCB, UK)
Robin Ketteler (LMCB, UK)
Janos Kriston-Vizi (LMCB, UK)
Paul Gissen (ICH, UCL, UK)
Karen Page (UCL, UK)
Anna Randi (Imperial College, UK)
Tom Nightingale (QMUL, UK)
 Close
Close


