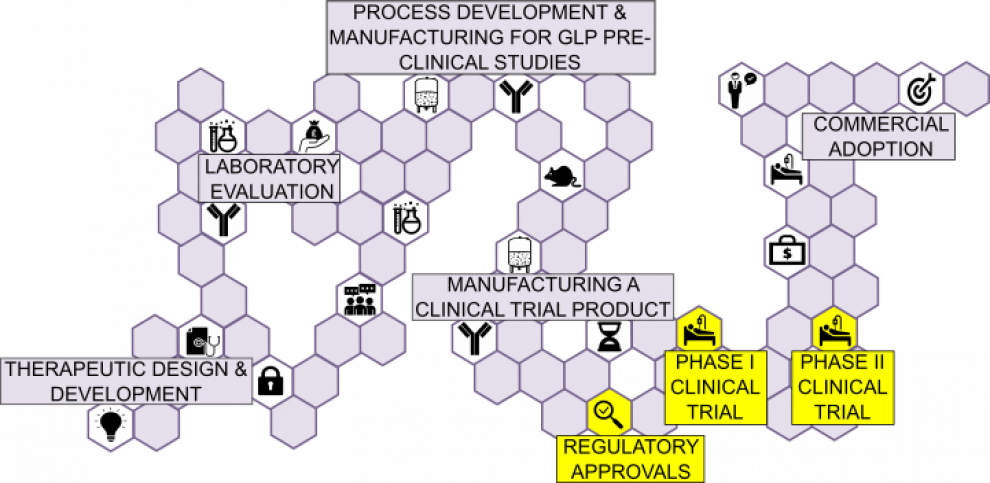
Drug developers are required to conduct clinical trials to establish the safety of a new biologics therapeutic in human subjects.
 PHASE I CLINICAL TRIAL:
PHASE I CLINICAL TRIAL:
First-in-man Phase I clinical trials assess the safety of a new medicinal product. They can also encompass a secondary objective of assessing efficacy, if administered to a patients population, and can be compared to standard of care practice.
Phase I clinical biologics trials must be compliant with Good Clinical Practice (GCP) and are typically dose escalation studies to establish maximum tolerated dose without toxicity.
PHASE II CLINICAL TRIAL:
Phase II clinical trials objective is to assess safety and efficacy of biologic. Protocol is based upon anticipated real world dosing regimen.
Phase II clinical biologics trials must:
- Be compliant with Good Clinical Practice (GCP)
- Be conducted on target patient population
UCL Support:
The Joint Research Office (JRO) or Comprehensive Clinical Trials Unit (CCTU) collaborates with researchers to design, conduct, analyse and publish clinical trials and other well-designed studies.
The JRO or CCTU will support and oversee investigator-initiated, clinical evaluation of biologics development programs at UCL:
- http://www.ucl.ac.uk/jro/academic-clinical-trials/sponsorship-clinical-trials
- http://www.ucl.ac.uk/jro/approvals/research-network-coordinators-contact
The CCTU is a registered CTU and coordinates multi-centre clinical trials (i.e. has overall responsibility for the design, development, recruitment, data management and analysis of trials) with robust systems to ensure the highest quality standards of conduct and delivery of clinical trials.
 REGULATORY APPROVALS:
REGULATORY APPROVALS:
Engaging with Regulators in advance is extremely important in order to identify and address potential hurdles to translation. Ensuring rigorous compliance of UCL researchers and contractors to required international standards.
UCL Support:
The Joint Research Office (JRO) and Translational Research Office (TRG) support developers in seeking local, national & international approvals (Ethics, HRA, MHRA, R&D).
 Close
Close

