One of the important experiments used in establishing the structure of unknown (natural) compounds is abbreviated as ADEQUATE. In their implementation on Bruker instruments, m,n-ADEQUATE experiments provide correlations of carbon and proton chemical shifts via successive mJCH and nJCC couplings. The simplest experiment of this type is 1,1-ADEQUATE, where H-C-C correlations are revealed via one 1JCH and one 1JCC coupling. The spectrum of menthol is shown below:
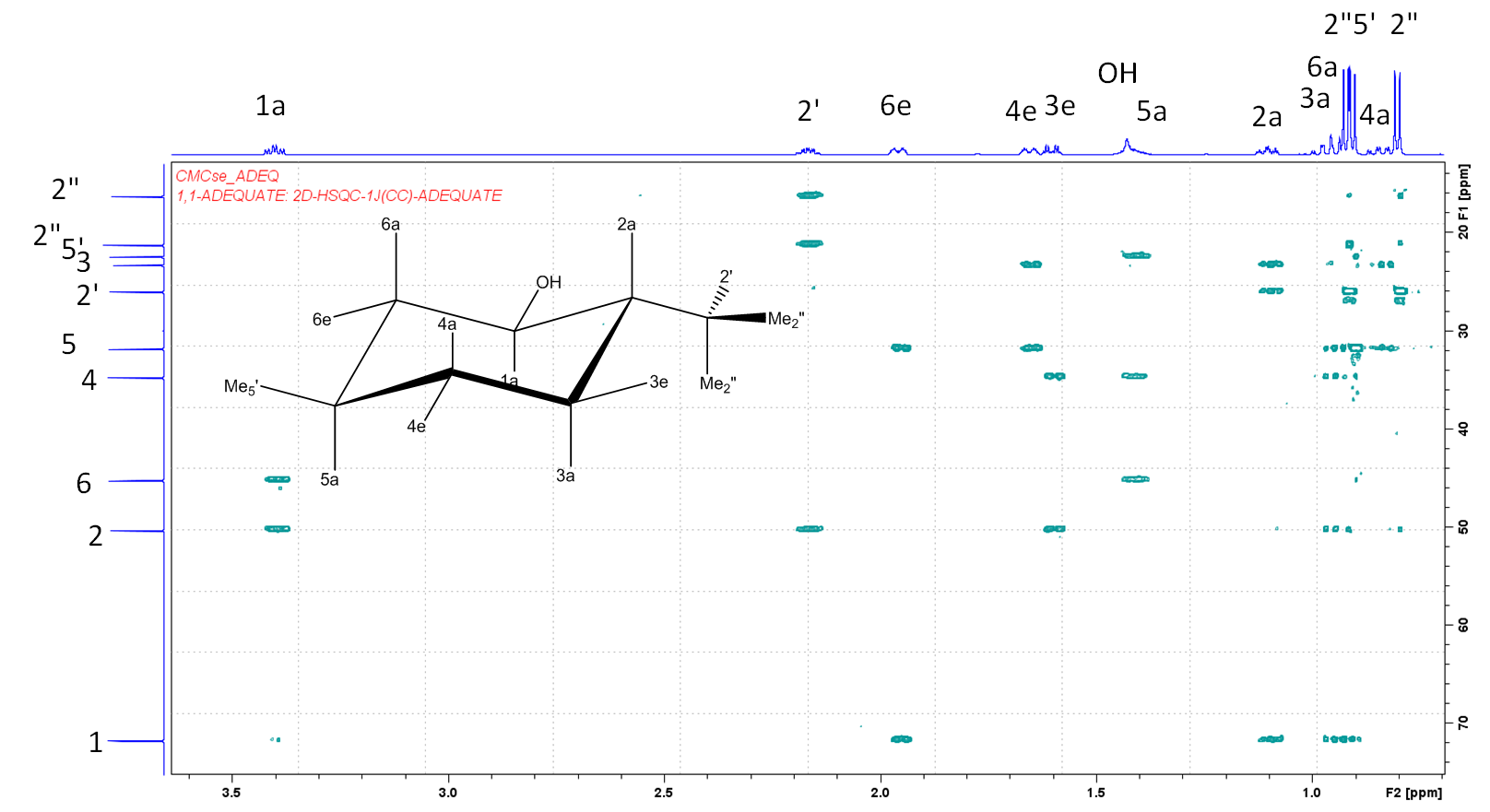
In the above 1,1-ADEQUATE spectrum, acquired for 400 mM solution in CDCl3 in 3 hours (600 MHz/DCH cryoprobe), we see correlations of carbon 2 with protons 1a, 2', 3e and 3a. There are no other protons which are two bonds away from carbon 2. The same type of correlations are also observed in the 1,N-ADEQUATE spectrum of menthol below (in blue below), but additionally we can also detect H-C-C-C-C correlations (in green):
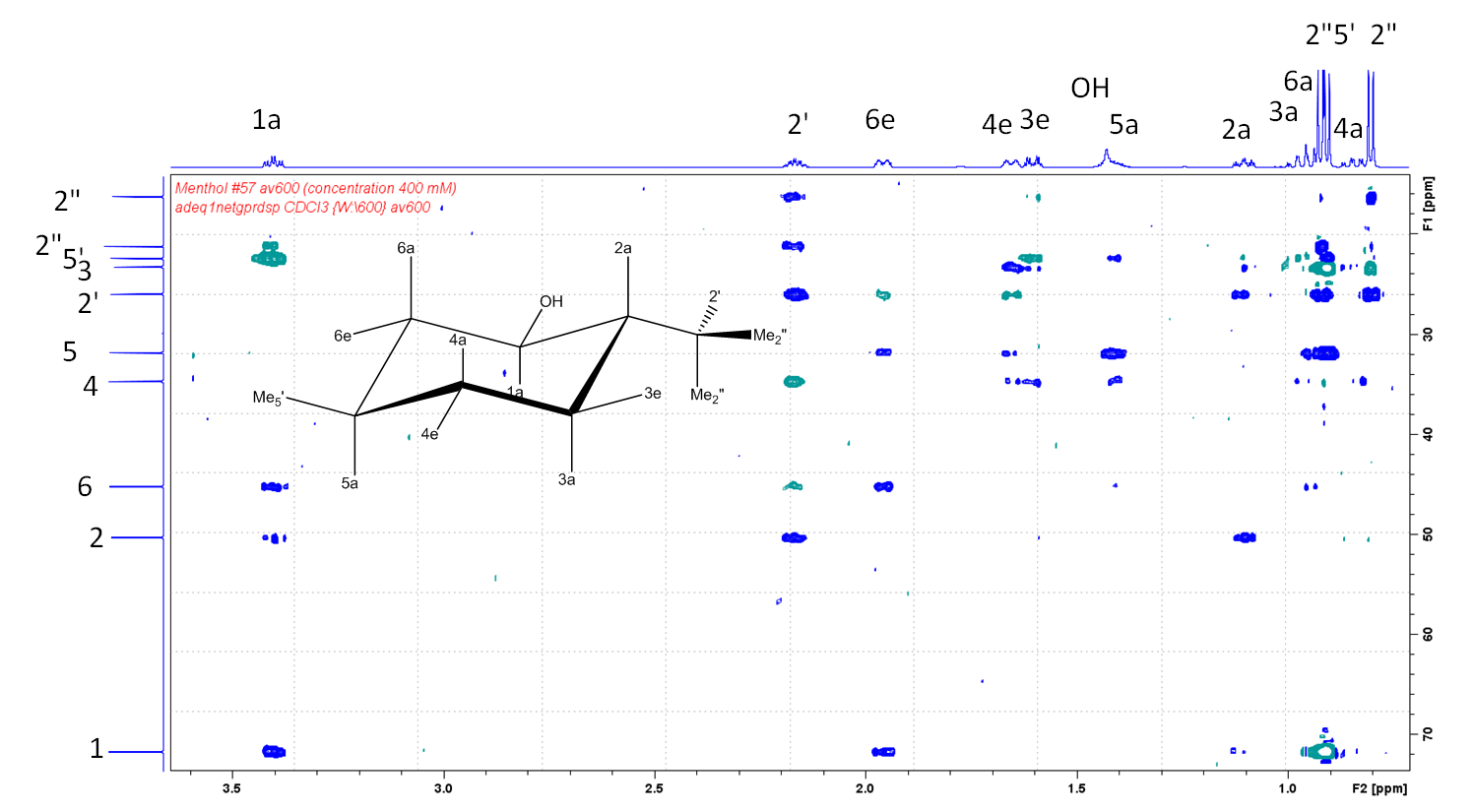
However, not all the 1,1- or 1,3-ADEQUATE cross-peaks are observed in the spectrum above (recorded for 400 mM solution in CDCl3 for 14 hours on 600 MHz instrument with a DCH cryoprobe). This is mainly due to the relatively poor sensitivity of 1,N-ADEQUATE experiments. The 1,1-ADEQUATE experiment is significantly more sensitive in this regard (3 hours of acquisition).
Note that the observation of all 1,1-ADEQUATE cross-peaks provides the same type of information as INADEQUATE. However, you may still fail to deduce the structure of the unknown compound if there are too many heteroatoms in your molecule (search for "Crews rule").
 Close
Close

