Frequently, second-order effects are observed in NMR spectra which could obscure the analysis or the verification of the product of a reaction. Some examples are included below, which required additional numerical analysis using spectral simulations and lineshape fittings.
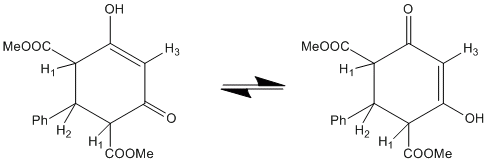
1) Due to fast exchange in the NMR time scale, protons denoted as H1 below become equivalent:
Signals of cyclic protons H1 and H2 show signs of second-order effects in CD3OD (400 MHz, 298 K) and for accurate determination of values of J couplings, full lineshape analysis was carried out using gNMR. In the screenshot shown below the experimental spectrum is shown in red and the fitted spectrum is shown in black:
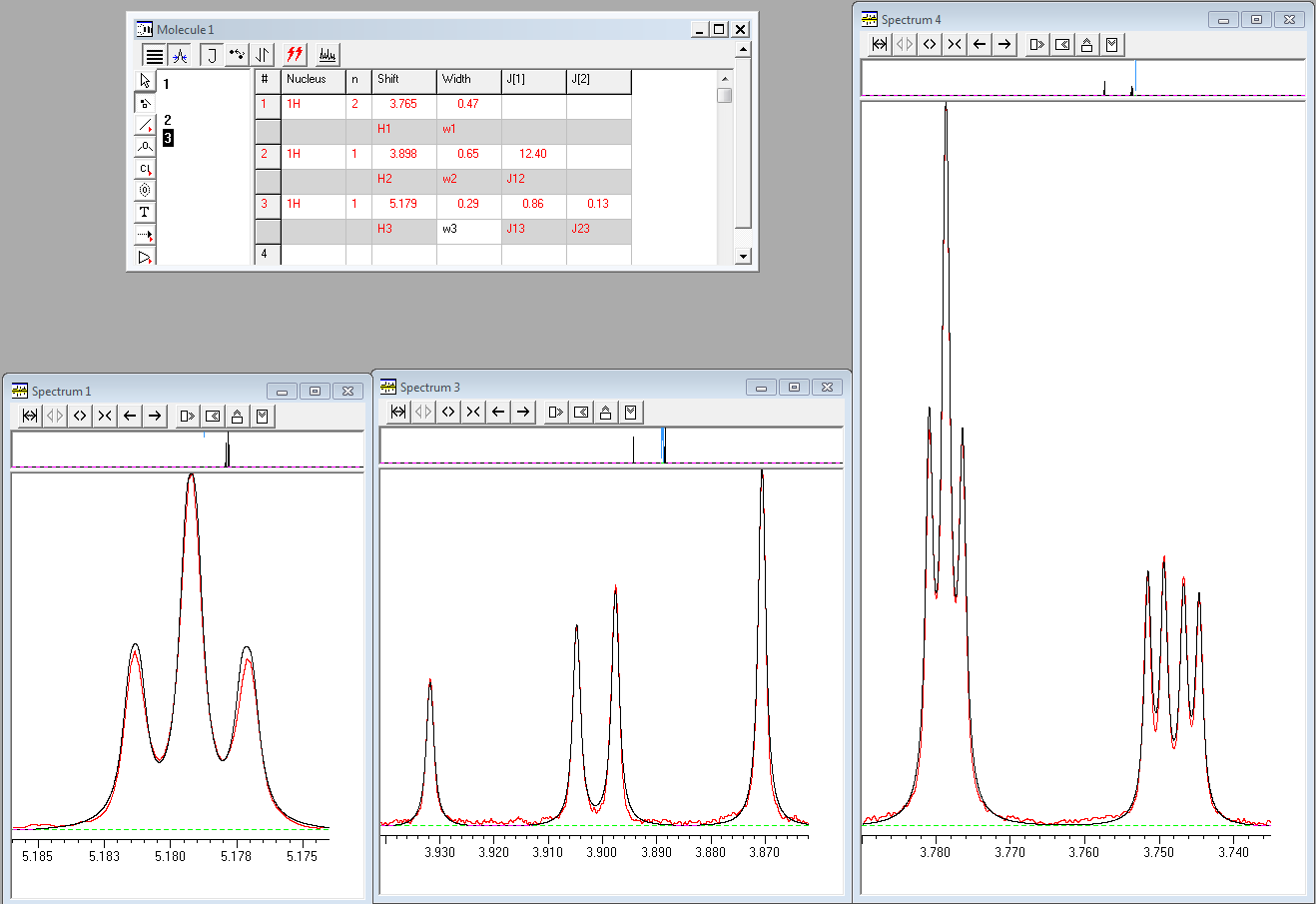
While proton H2 appears as a doublet of doublets with splittings of 10.8 and 13.6 Hz at 3.90 ppm, the analysis shows that none of these is the true value of the 3J(H1,H2) coupling. The value of this coupling determined from the full lineshape analysis is 12.40 Hz.
2) Both 1H and 15N NMR spectra of urea with two 15N labelled nitrogens (urea-15N2) in DMSO-d6 show unusual lineshapes, which attracted the attention of many in the mailing list of NMR spectroscopists, AMMRL. While experimental spectra can be fitted using lineshape simulations, the agreement for outer components of the "triplet" observed in the 15N spectrum was relatively poor. In the gNMR screenshot below the experimental 15N spectrum is shown in blue, the experimental 1H spectrum is shown in red and iteratively fitted lineshapes are shown in black:
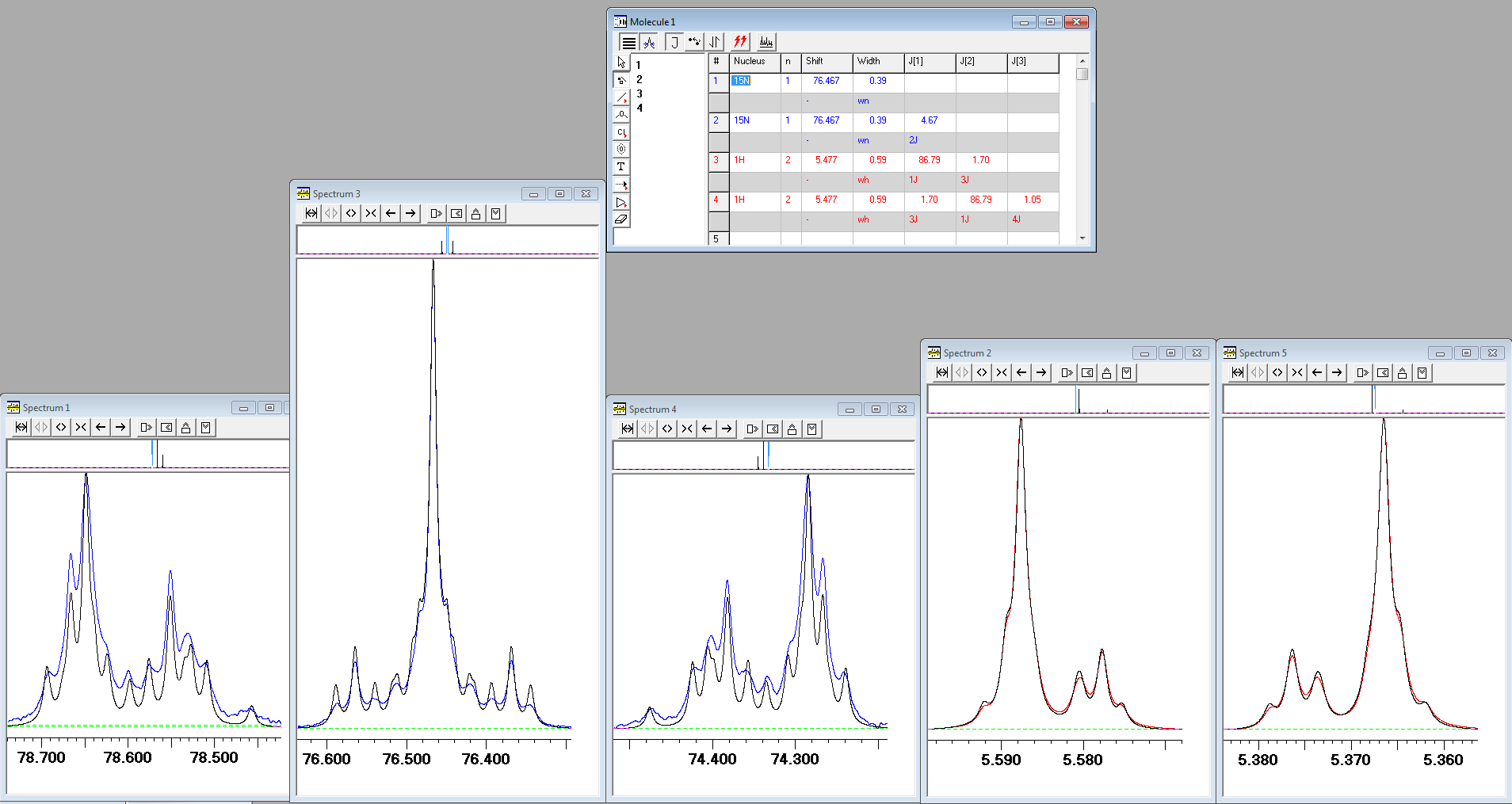
After much search for the reason of such disagreement, the solution was found by your humble servant. It turned out that the NH2 protons of urea are exchanging slowly with protons of water present in DMSO-d6. On inclusion the intermolecular exchange into lineshape fittings, the agreement between the calculated and experimental spectra improved considerably (click here for high-resolution image):
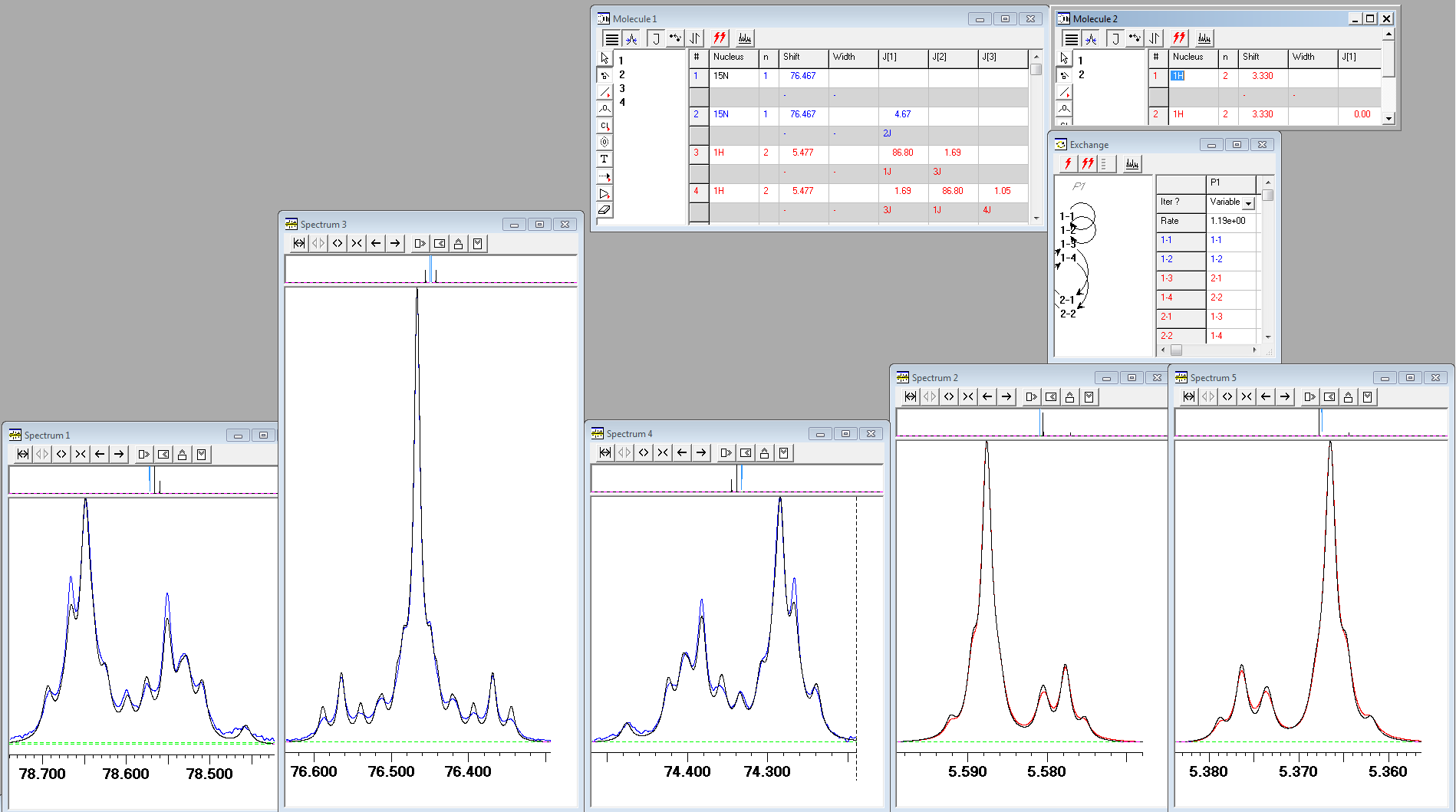
Note that the rotation around the N-C bond of urea in DMSO solution is very fast in the NMR time scale at room temperature, thus equalising "equatorial" and "axial" urea protons defined relative to the direction of the carbonyl bond.
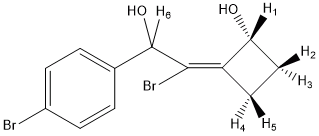
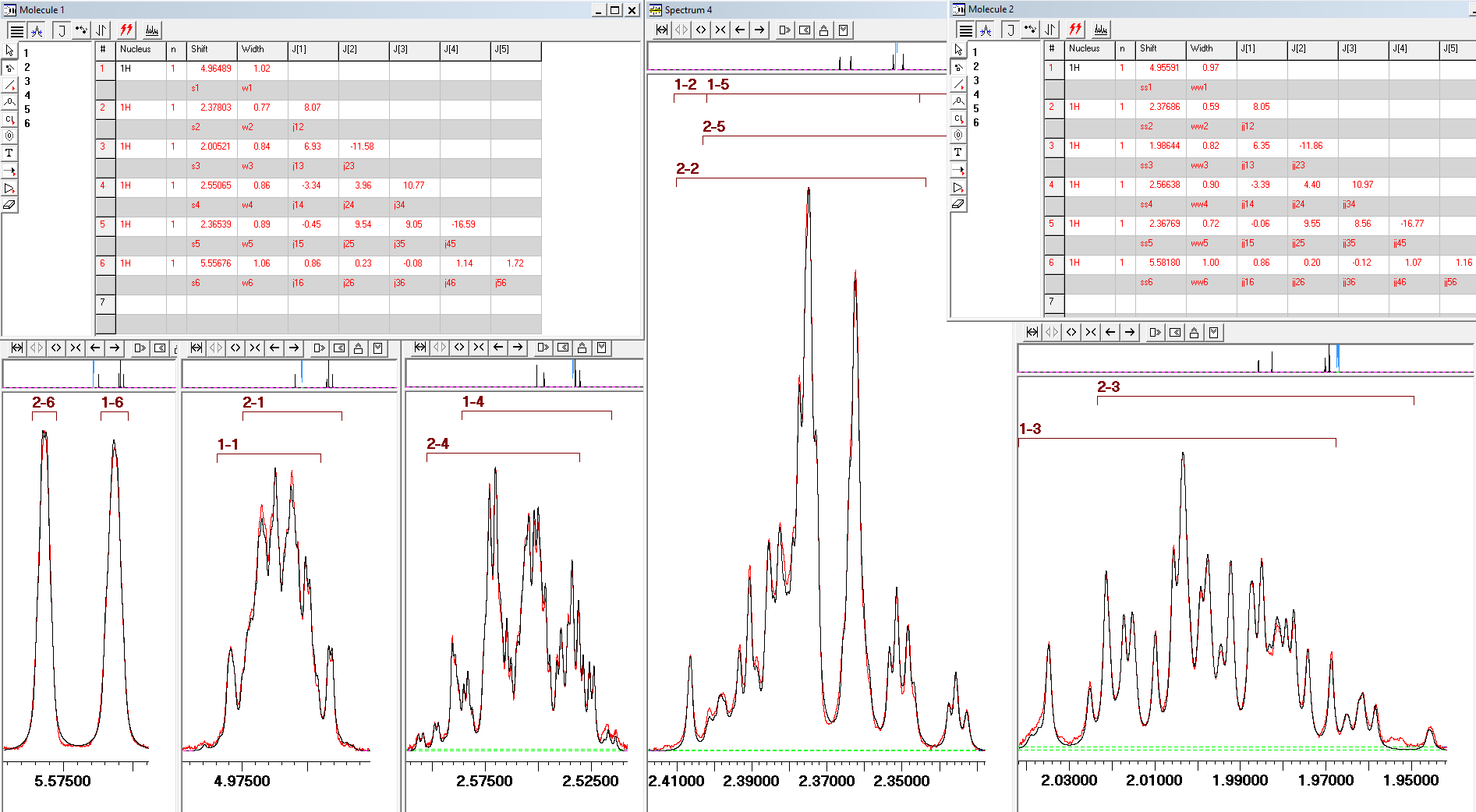
3) In certain cases, the only way to extract chemical shifts and J couplings is through full line shape analysis. The 600 MHz 1H NMR spectrum of the ~1:1 mixture of two diastereomers of the shown below cyclobutane derivative was analysed in this manner in order to determine NMR parameters.
 Close
Close

