Within cell and gene therapy projects, it is a common need to contract out some of the work to CRO's externally, outside of UCL.
 GMP MANUFACTURING:
GMP MANUFACTURING:

Outsourced work – exceptional costs?
UCL procurement rules for work over £50k.
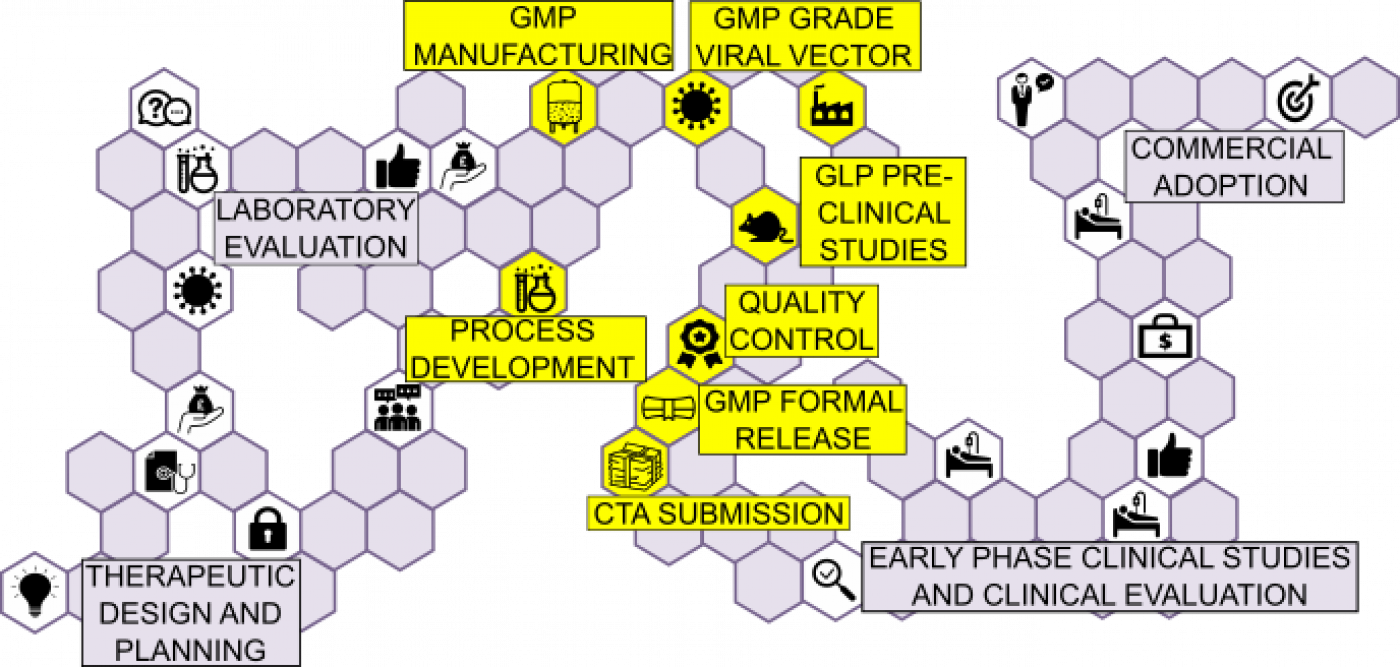
CRO manufacturer will usually operate under a Master Services Agreement (MSA) and Schedule of Work (SoW) which defines terms, scope, objectives, timelines and deliverables of the work package. If a collaborator rather than a fee for service supplier, there will also be a collaboration agreement.
- Engage early to reduce risk– ensure due diligence with all CROs completed. Slots for CRO: check details – delays etc
- Contract agreements for preclinical GLP Toxicology & GMP CROs, CTUs – secure SoWs - all agreements in conjunction with UCL Research Services, TRO and UCLB
- GMP batches ready – supply chain secured – stability and potency ongoing for duration of studies
UCL Support:
The UCL Translational Research Office (TRG)'s expertise and knowledge of the cell and gene therapy field means that they know which GMP manufacturers are in the best position to help and allow you to start the conversation with them.
 CLINICAL TRIALS APPLICATION (CTA) SUBMISSION:
CLINICAL TRIALS APPLICATION (CTA) SUBMISSION:
All documentation relating to manufacturer, suppliers needs to be included for a CTA - ie, IMPD/IB, HRA – MHRA class gene therapies as ATMPs (Directive 2001/83/EC, ATMP regulation 1394/2007 applies)
Committee for Advanced Therapies (CAT) oversee all marketing authorisations
UCL Support:
There are several clinical trial units that the UCL Translational Research Group can liase with, including:
- Cancer Research UK & UCL Cancer Trials Centre
- Comprehensive Clinical Trials Unit at UCL
- Medical Research Council Clinical Trials Unit at UCL
- PRIMENT Clinical Trials Unit
The TRG work with the Joint Research Office to handle clinical trial related work.
 Close
Close

