Where research is taking place on NHS premises and/or involves NHS patients or staff then the Trust (UCLH) needs to ensure they have the capacity and capability to undertake the study. The Trust also needs to ensure adequate contracts and any cost implications are covered.
The Health Research Authority (HRA) refers to this process as "Assess, Arrange and Confirm". At UCLH, we also use the term "feasibility" to cover this process.
For studies at UCLH, the Joint Research Office (JRO) will coordinate the Assess, Arrange and Confirm process.
How to apply for Assess, Arrange and Confirm at UCLH
To initiate an AAC review, researchers should provide the JRO with a minimum document set. The minimum document set is defined here.
The documents should be sent to uclh.randd@nhs.net along with a completed checklist.
What happens next?
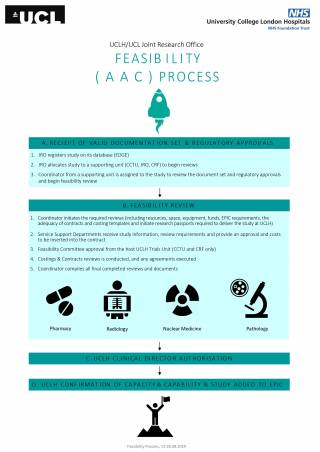
Your study will be registered on the UCLH database by the JRO team and allocated to one of three supporting units which manage the AAC process. These supporting units are the JRO, Clinical Research Facility and the Cancer Clinical Trials Unit at UCLH. The PI and sponsor will be notified via an email of the point of contact in the relevant unit.
- The supporting unit will review the study (conduct a feasibility assessment). If there are any immediate issues, they will notify the PI and the sponsor within 3 working days
- The supporting unit will then initiate reviews with other departments by sending any Services Support Department (SSD) forms to the JRO and initiating costing and contracting
- The JRO will then forward any SSD reviews to the relevant SSD leads. The SSDs will notify the PI, unit, and sponsor of any likely issues within 3 working days
- A target timeline for approval will then be discussed and agreed between the supporting Unit, the PI and the sponsor
- The JRO costing team (Cost Accountant) will support the PI and Unit coordinator with completing the necessary costing templates and cost related documents. SSDs will provide their input to these documents
- The JRO contracts team (Contracts Manager) will support the PI and unit coordinator with completing any contracts and discussing modifications with the PI and Sponsor
- The Clinical Director at UCLH will be asked to approve the study
- Contracts will be signed through the JRO admin and Contracts team
The JRO will issue a confirmation the study can proceed at UCLH (this is called the decision to deliver and will sent from uclh.randd@nhs.net)
View a flowchart of the feasibility process.
Conditions and timelines
- Incomplete submissions will be deemed as invalid and will be returned and the review will not progress.
- Should the minimum document set be received, the aim is to confirm AAC either within 40 days (applicable for most studies) or to the timeline agreed with the sponsor (minimum 40 days for complex studies).
- The anticipated timeline may increase where a new technique or methodology is identified, or complex set-up arrangements are required.
- Should any issues or complexities (which could impact the timeline) be identified, the PI and sponsor will be notified within 3 working days. Service leads are also made aware.
- Unless identified and agreed, the target turnaround time for SSD and costing reviews are 10 working days. Sponsors will be advised that the target contract turn is 10 working days and therefore their response to queries raised by UCLH are requested within 3 working days.
- Turnaround times are subject to having received the required documents (completed), no-changes being made after the documents are received and a prompt response on queries from all parties.
- The timeline will be agreed between the unit, the Sponsor and PI. Any change to this will be discussed in good time by the Unit with the Sponsor and PI.
- In order for UCLH to reach the timelines it agrees for each study, we request that PI’s and sponsors respond to queries in good time. We request a response to queries within 3 working days.
Studies which are managed through the Cancer Clinical Trials Unit, or the Clinical Research Facilities may require an internal approval (known as a feasibility committee). These reviews will be managed within the agreed turnaround time for set-up. Further information will be provided by the coordinator supporting the study. Should you require any information regarding the Cancer Clinical Trials Unit, please email: uclh.cctusetup@nhs.net and the Clinical Research Facility.
Queries relating to the progress of a study should be directed to the assigned coordinator from the supporting unit. Researchers who wish to contact the leads for AAC directly (Research Governance Manager, Finance or Contracts Lead in the JRO) should email: uclh.jro-communications@nhs.net with the subject line: AAC study query
Studies which use anonymous data from UCLH (only) may be eligible for a combined sponsorship and AAC process called the DAP-R. DAP-R is relevant for studies sponsored by UCL or UCLH only. See our Data pathways at UCL webpage for further information on the DAP-R and accessing data at UCLH.
 Close
Close

