MOTOR NEURON DISEASES
Motor neuron diseases (MNDs) are common and incurable, and arise when the motor neurons that extend from the brain into the spinal cord and out to the muscles, degenerate and die. Spinal muscular atrophy, one form of MND, is the biggest single genetic killer of children, and amyotrophic lateral sclerosis (ALS) is an adult form of MND that arises in mid-life. Although single genes are known to cause MNDs, it is not clear how these mutant genes exert their effects or what other genes can modify the pathways involved. Many different cellular systems are affected, including those involving RNA metabolism. We create and analyse bespoke models to drill into pathomechanism, and we collaborate with a number of groups, including those of Pietro Fratta, Adrian Isaacs, Linda Greensmith and Giampietro Schiavo at UCL and Tom Cunningham at MRC Harwell to analyse these models.
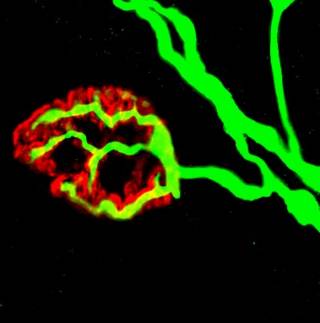
The neuromuscular junction (NMJ) were stained with neurofilament/synaptic vesicle (in green) and α-Bungarotoxin (in red) collected from the lumbrical muscle of a wildtype mice. The figure depicts a healthy NMJ branching from an axon with evident coverage of the postsynaptic side.
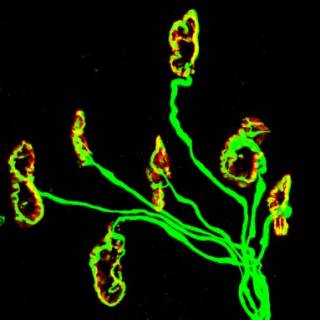
The neuromuscular junctions (NMJ) were stained with neurofilament/synaptic vesicle (in green) and α-Bungarotoxin (in red) collected from the lumbrical muscle of a wildtype mice. The figure depicts healthy NMJs with prominent innervation of the motor endplate.
X-LINKED DYSTONIA PARKINSONISM
In a separate project we are collaborating with the Bradley lab at the Sanger Institute to create a new mouse model to understand the disorder X-linked Dystonia Parkinsonism (XDP). This is a novel genome engineering project in which we have to knock in >175 kb of human DNA into the mouse genome, to recreate the specific mutation that was recently discovered to be causative. XDP is an relentless and aggressive early onset movement disorder arising from a single mutation in the TAF1 gene.
DOWN SYNDROME
We are also working with mouse models of Down syndrome; all this research is carried out with our long-term collaborator Professor Victor Tybulewicz of the Francis Crick Institute.
Down syndrome is the most common known genetic form of intellectual disability and the most common genetic factor for susceptibility to Alzheimer disease (AD). Down syndrome arises from having three copies of human chromosome 21 and commonly results in typical AD pathology within the brain by the age of 40, greatly increasing the risk of developing dementia. The novel DS mouse models generated by our group allow the contribution of different genes on human chromosome 21 to be analysed – furthering our understanding of the development of AD in the Down syndrome population.
This research is being carried out with Dr Frances Wiseman, an ARUK Fellow.
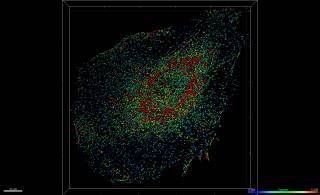
3D mask of Rab5 endosomes in Mouse Embryonic Fibroblast (MEF), colour-coded by volume. Figure realised by Dale Moulding and Claudia Cannavo.
In addition, we are analysing our mouse models of DS for specific cognitive and electrophysiological changes, in collaboration with Professor Matthew Walker of UCL. This will give us new insight into disrupted processes in DS, and potentially the underlying genes.
We are also members of the LonDownS Consortium that takes a multidisciplinary approach to defining the connection between Down syndrome and Alzheimer disease.
http://www.ucl.ac.uk/london-down-syndrome-consortium
MRC HARWELL
We have many collaborations world-wide in order to maximise what we learn from each of our bespoke mouse models. We have particularly close ties with MRC Harwell and the Laboratory for Mouse Models of Neurodegeneration, run by Dr Thomas Cunningham.
https://www.har.mrc.ac.uk/news/research-spotlight-dr-thomas-cunningham/
https://www.har.mrc.ac.uk/news/humanising-mice-to-enable-modelling-of-neurodegenerative-diseases/
 Close
Close

