Our humanitarian intention is to share the UCL-Ventura designs and manufacturing instructions to help public health efforts during the Covid-19 pandemic emergency.
The UCL-Ventura breathing aid is a Continuous Positive Airway Pressure (CPAP) device that supports patients with breathing difficulties.This CPAP has been developed as a collaboration between UCL, University College London Hospital and Mercedes-AMG High Performance Powertrains. The design is based on an existing off-patent CPAP system that has been further modified to optimise oxygen consumption.
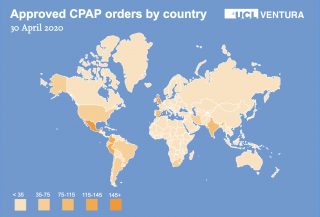
We released the designs and manufacturing instructions for the device on Tuesday 7 April, as of May 2020 we had approved over 1850 requests from 105 countries spanning Europe, Asia, Africa, Americas and Australasia. We have since supported many of these countries and teams through translation, manufacture and distribution.
UCL-Venturas are being used in 29 countries across the globe.
How do I request the manufactured devices interntionally?
Please contact CPAPcovid19@ucl.ac.uk if you do not have access to the manufacturing capabilities to produce devices using the designs and manufacturing instructions, and you have a specific request for devices to be supplied internationally.
How do I request the designs and manufacturing instructions for the device?
We are releasing design and manufacturing instructions for free for the UCL-Ventura CPAP design to support healthcare systems around the world to provide respiratory support for patients with Covid-19.
The design and manufacture instructions package for this CPAP includes the following:
- Manufacturing drawings
- System schematics and characteristics
- Bill of materials and type of manufacturing machines used for CPAP production
- Development tests information
- Assembly procedures, including build tooling requirements
- Test procedure and pass-off protocol
This CPAP has been approved for manufacture by UCL by UK regulators, the Medicines and Healthcare products Regulatory Agency (MHRA) under special conditions. These conditions state that this is a non-CE marked CPAP, given approval for use in the NHS for the interest of public health protection under the Covid-19 pandemic emergency.
National Health Service (NHS) guidance for the role and use of non-invasive respiratory support (including CPAP) in adult patients with COVID-19 is available on the NHS website here. Further UK guidance on the use of personal protection equipment (PPE) is available on the Government website here. World Health Organisation (WHO) guidance on resource planning for COVID-19, including critical items (PPE, diagnostic equipment, clinical care equipment) is available on the WHO website here.
Any manufacture and use of this CPAP by third parties must require the third party to have local regulatory approval in place, as required in the third party’s own country and must fully comply with any stipulated conditions, laws and regulations that ensure full patient safety.
The technical specifications for this CPAP are being shared for humanitarian purposes, to help support the international community addressing pressing demands to care for Covid-19 patients. This is not a business venture. There is an expectation that those using these specifications to manufacture these devices follow the same guiding principles.
The instructions for manufacture should be followed precisely to ensure quality and safety, with no deviations or substitutions.
All hospitals must work with their O2 engineering teams and ascertain their VIE outflow and downstream flows and pressures to specific ward areas before deploying these devices. Healthy patient evaluation data indicates O2 flow rates of 11, 14, 17 l/min for inhaled oxygen (FiO2) levels of 30, 40, 60%, respectively for calm breathing and O2 flow rates of 14, 25 and 46 l/min at FiO2 of 30, 40, 60%, respectively for heavy breathing.
Access to this information will be provided at no cost to governments, relevant industry manufacturers, academics and health experts. To help ensure relevance and legitimacy, users must make requests using their work, university or institution email address.
Requests can be made directly at: covid19research.uclb.com/product/ucl-cpap. Please contact ihecovid19response@ucl.ac.uk with any queries. If you have a technical query please read these Frequently Accessed Questions (FAQs) first:
Please be aware that we have been inundated with requests so may be unable to respond immediately. But, we will work to respond to requests as soon as possible.
 Close
Close