Our iBSc Clinical Trials offers you a unique opportunity to learn the important concepts and practices underpinning appropriate clinical trial design, conduct, analysis, and interpretation of trials.
Learn more about the iBSc Clinical Trials from our staff in the video below.
Clinical trials are essential to help us discover whether new healthcare interventions improve outcomes for patients and the public. Our iBSc Clinical Trials offers you a unique opportunity to learn the important concepts and practices underpinning appropriate clinical trial design, conduct, analysis, and the interpretation and reporting of results.
The Institute of Clinical Trials and Methodology (ICTM) leads many UK and international trials which influence clinical practice. The ICTM has the largest group of trialists - approximately 400 - in Europe. The Institute is part of the Faculty of Population Health Sciences in the UCL School of Life and Medical Sciences.
In this one-year programme you will explore the role of the clinician in clinical trials. The programme will help shape your approach to clinical trials and the importance of clinical evidence for the rest of your MBBS programme and in your future career.
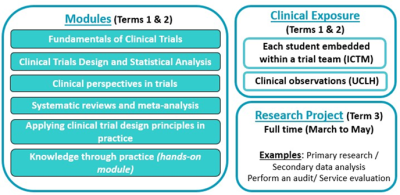
The first term of the programme is designed to provide you with a firm foundation in clinical trials from the research idea, through trial design, set up and conduct, to analysis and reporting of results. The need for clinical trials to improve upon standards of care will be clearly outlined during the first term. You will gain sufficient practical knowledge of statistics to enable you to critically evaluate the evidence base. You will later apply these skills when completing your research project.
In term two, you will be guided through real world examples of clinical trials to explore how the principles you have learned in term one apply to clinical trials in practice. You will learn about the purpose and conduct of systematic reviews and meta-analyses, and their application.
In terms two and three you will also undertake an independent research project which will enable you to carry out a literature review, perform an independent analysis of your findings and report your results in a dissertation.
Thank you for your interest in our programme. For further information please contact:
- Programme Directors, Dr Aleksandra Gentry-Maharaj a.gentry-maharaj@ucl.ac.uk and Dr Alejandro Arenas-Pinto a.arenas-pinto@ucl.ac.uk
- Programme Administrator, Ms Rahima Khanum ictm.pgtct@ucl.ac.uk
Visit the iBSc website for more information.

 Close
Close

