Cardiovascular diseases are the leading cause of death globally. Every year approximately 8 million people die from coronary heart disease and 7 million from stroke. Although treatments are improving, mortality rates continue to increase and it is imperative that new therapies are developed. Imaging is integral to diagnosis and prognosis of cardiovascular disease. In CABI we utilise advanced biomedical imaging techniques to monitor disease progression and efficacy of novel therapeutics in live rodent models of cardiovascular disease. This research identifies new treatment strategies and novel physiological and pathological pathways.
Imaging cardiac morphology and function
Heart structure and function are altered by disease. Using cine-MRI and ultrasound we can accurately identify changes in contractility, chamber volume, mass and aortic flow, while vascular structures can be assessed using contrast enhanced CT.
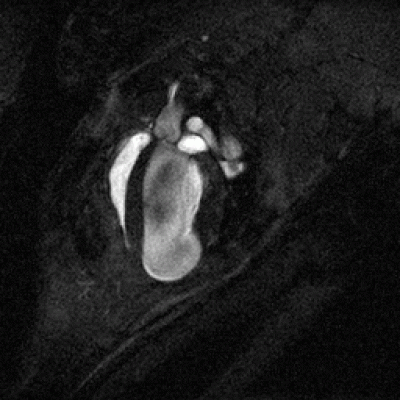
CINE-MRI
An animation produced by CINE-MRI of an infarcted mouse heart
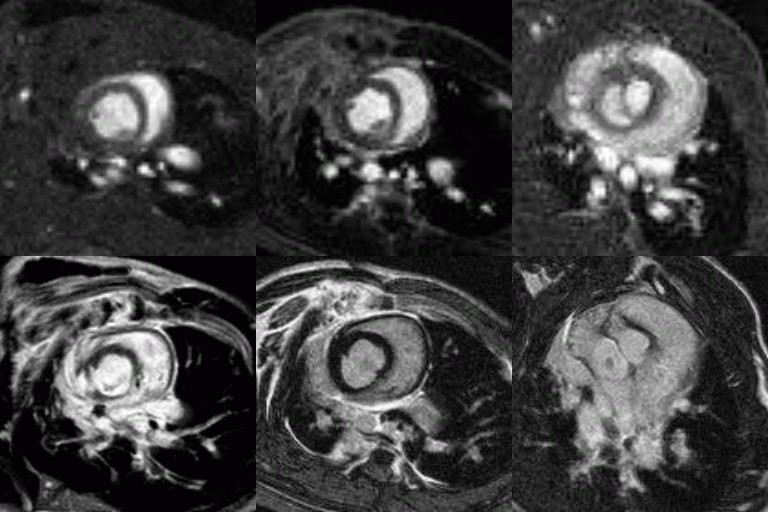
Viability
Top row shows CINE-MRI at 3 progressing time-points (columns). Bottom row images show late gadolinium enhancement for viability imaging. The evolution of the infarcted scar tissue and ventricular dilation over time is apparent from left to right
Imaging cardiac viability and fibrosis
Ischemia rapidly leads to cell death and subsequent fibrosis. These changes can be indirectly assessed by MRI after injection of Gadolinium based contrast agents. In myocardial infarction the contrast agent accumulates within the infarcted tissue, enhancing the MRI signal from the effected region, while in pathologies such as hypertension and aortic stenosis that result in diffuse accumulation of fibrosis, the increased contrast accumulation can be detected using T1 mapping. Positron emission tomography (PET) can also be used to assess tissue viability after infusion of 18F-FDG - a radioactive glucose analogue. Damaged myocardium has lower metabolic rate meaning that the accumulation of glucose is lower in affected regions.
Imaging cardiac perfusion
The rate of blood flow within the myocardium can dramatically affect cardiac function. We use arterial spin labelling (ASL) MRI and first pass dynamic contrast enhancement (DCE) MRI to quantify perfusion within hearts of animal models of cardiac disease.
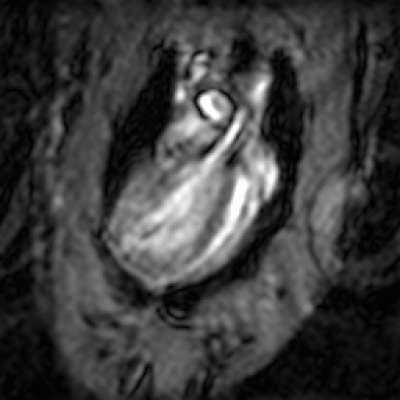
1st pass perfusion
Animation showing the perfusion of contrast agent in the mouse heart. he hyper-enhancement caused by the contrast can be seen moving first into the blood pool before perfusing the myocardium.
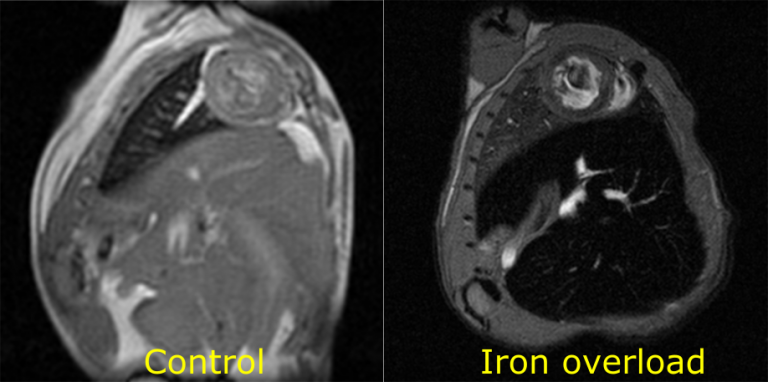
Iron overload
Pathological accumulation of iron in the heart is quantifiable through MRI. The reduction in signal intensity caused by the presence of iron is clear in the iron overload image
Imaging cardiac iron load
Beta thalassemia major results to accumulation of iron within the myocardium, and can lead to cardiac dysfunction. Iron accumulation can be assessed in patients using T2* MRI. We have developed preclinical MRI methods for monitoring iron accumulation and response to therapy in a mouse model of thalassemia.
Imaging cardiac ultrastructure
The helical array of myofibers that make up the structure of the heart are important for efficient contraction. Using diffusion tensor imaging (DTI) and 3D optical imaging, such as optical projection tomography (OPT) we can assess the alignment and disarray of myofibers within whole heart specimens ex vivo.
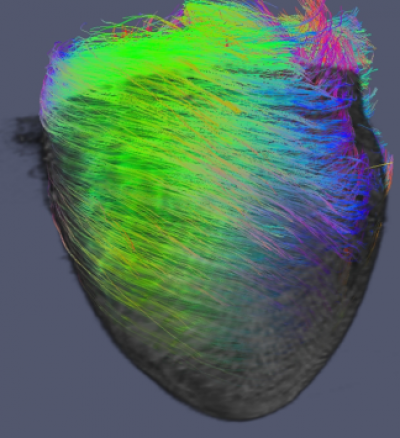
Imaging across scales
DT-MRI can be used to image myofibre orientation and OPT for superstructural anatomical information. Co-registering these image sets allows for complete quantification of cardiac architecture
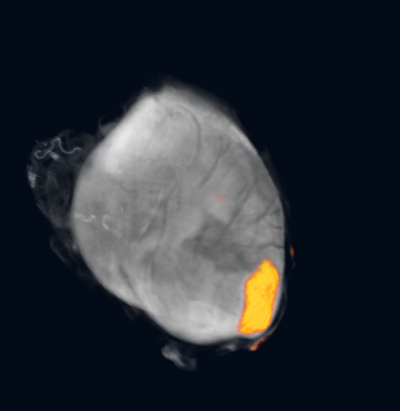
Imaging cardiac regeneration
Fluorescent stem cells form a patch to regenerate regions of myocardium
Imaging cardiac regeneration
One of the most promising therapies for myocardial infarction and prevention of heart disease is regenerative medicine. In CABI we investigate the benefits of cardiac and mesenchymal stem cell therapy for the heart. In addition to following the beneficial effects of cell therapy on cardiac function using the techniques mentioned above, we also track the fate of the grafted cells using genetic reporter genes such as luciferase for bioluminescence imaging or norepinephrine transporter for PET/SPECT; and with direct cell labelling with iron oxides, gold nanoparticles and radiotracers.
Retention of grafted stem cells within the heart is low, meaning that beneficial effects are small. We are using tissue engineering approaches to enhance cell engraftment. By grafting cells within nurturing hydrogels or seeded them onto scaffolds we aim to support stem cell survival and enhance regenerative effect. Specifically, we are developing methods that track the in vivo fate of the biomaterials and the stem cells, providing in vivo information on biomaterial location and degradation, as well as survival of cells within the engineered tissue.
Imaging stroke
MRI can be used to monitor changes in brain tissue after ischemic stroke. We use T2 weighted imaging to monitor vasogenic edema, and a combination of diffusion and perfusion imaging to identify the potentially salvageable penumbra of a stroke lesion.
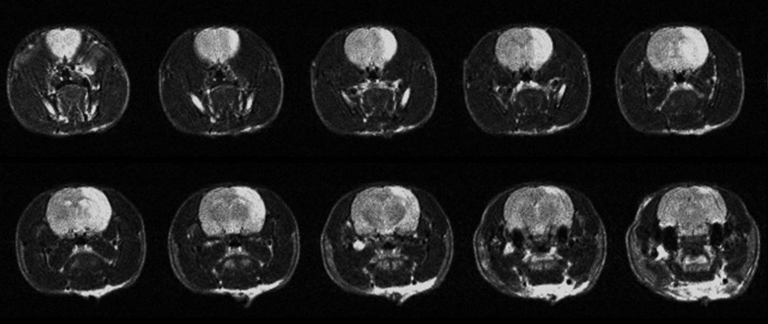
Imaging stroke
Using T2 weighted MRI, regions of the brain affected by stroke become hyper-enhanced
 Close
Close

