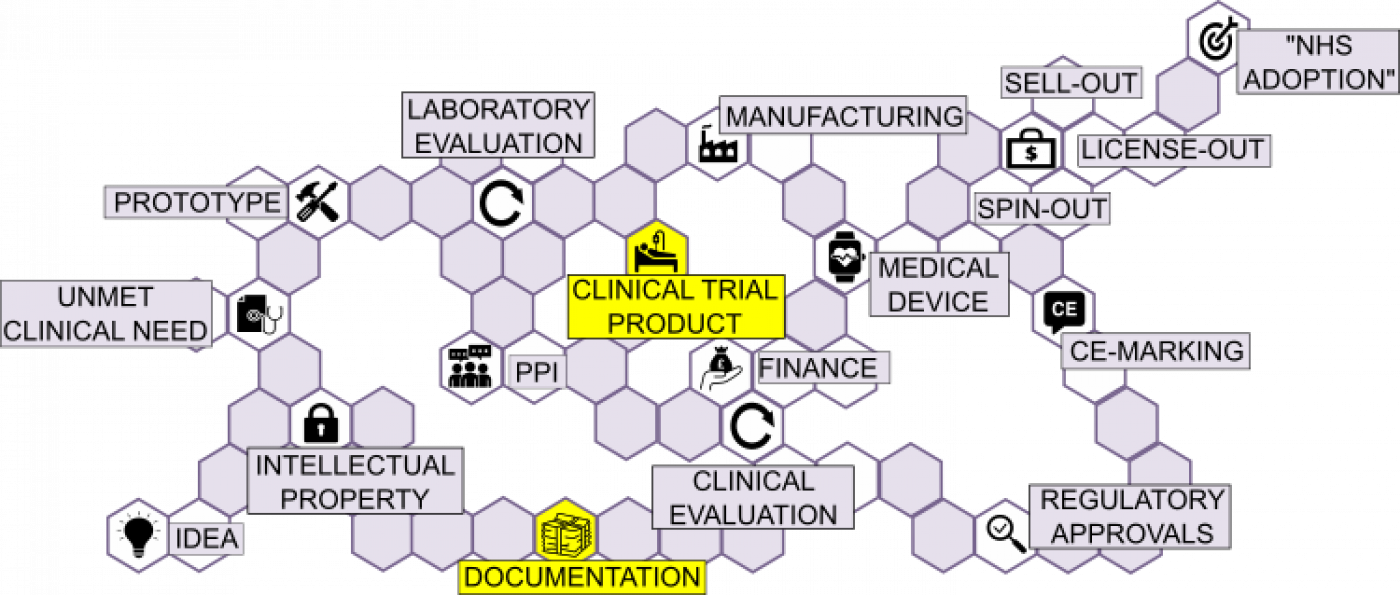
This section includes some important considerations around the manufacture of non-CE marked clinical trial product (CTP), intended for commercialisation.
CLINICAL TRIAL PRODUCT:
When transitioning to the manufacture of a ‘clinical prototype’, an agreement should be in place as to which organisation shall be the legal ‘Device Manufacturer’ (e.g. UCL, UCLH, RFH, MEH, responsible for the design, manufacture, packaging and labelling of a product for clinical trial, regardless of whether these operations are carried out by themselves or on its behalf by a 3rd party).
- Simple ‘aid to recovery’ app might be ‘manufactured’ by company set-up and owned by PI at UCL
- Complex ‘artificial organ’ likely to be outsourced in entirety to UCL-appointed device manufacturer
DOCUMENTATION:
All CMOs will be required to provide technical documentation to legal manufacturer or its appointed delegate. All documentation relating to manufacturer and suppliers shall be included in the medical device master file.
UCL Support:
- If employing a contract manufacturing organisation (CMO), UCL Research Services and the Joint Research Office (JRO) shall co-develop the Contract Manufacturing Agreement
- The JRO shall lead on the development of the Technical Agreement to ensure Good Manufacturing Practice (GMP) regulatory compliance (e.g. inspection of the CMO)
- If Device is progressed via a UCL spin-out then UCLB shall lead on all manufacturing agreements
 Close
Close