Highlighting important considerations during the "Therapeutic Design and Planning" stage of the Gene therapy translational pathway.
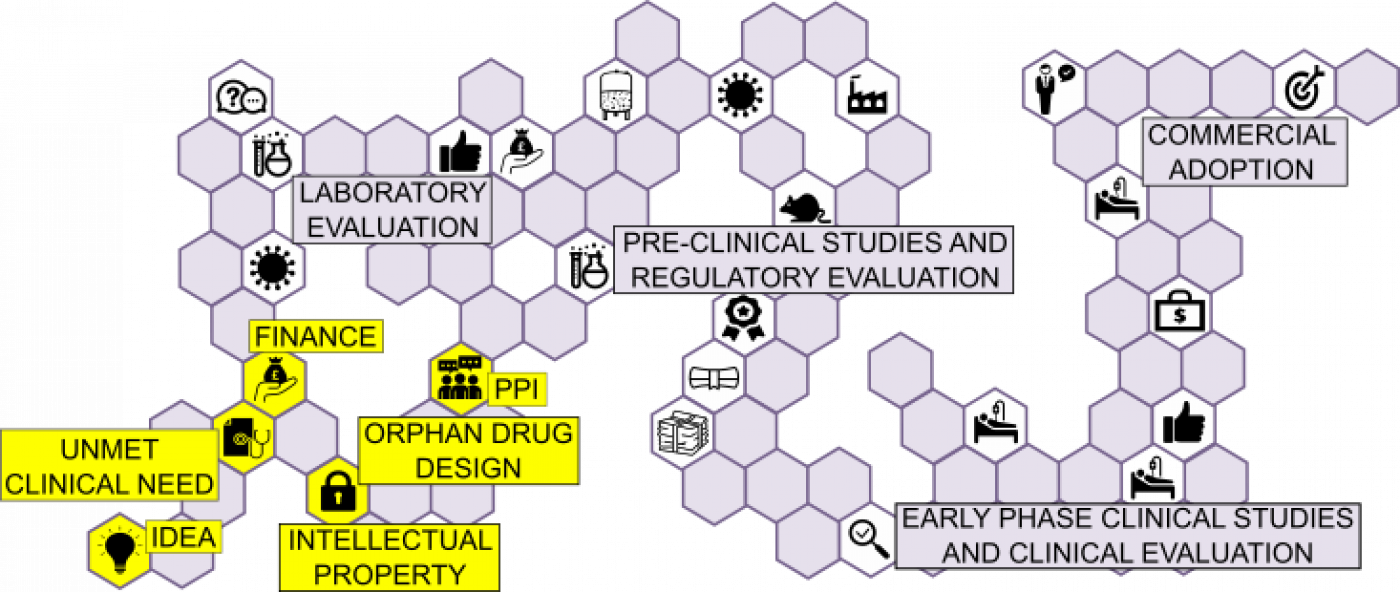
 IDEA:
IDEA:
Target Product Profile (TPP) – captures the ‘benefit’ of the proposed therapeutic and key considerations in the strategic development of the product (incl. technical, scientific and medical information required to satisfy key stakeholders (regulators and funders)).
Although advanced therapies can include viral and non-viral gene delivery, direct and ex vivo gene therapy, gene supplementation, gene editing and CAR-T cells, AAV is the predominant viral vector used globally with the greatest potential for gene therapy, especially for monogenic loss-of-function diseases. The viral vector used is dependent on the project and the desired nature of the therapeutic. For example:
| Characteristic | AAV | Lentivirus |
|---|---|---|
| Genetic Material | ssDNA | ssRNA |
| Coat | Naked | Enveloped |
| Packaging Capacity | 4.7 kb | 8 kb |
| Tropism/Infection | Serotype specific tropism | Broad |
| Immunogenicity | Low | High |
| Host genome interaction | Integrating/non-integrating | Integrating |
| Transgene expression | Potentially long lasting | Long lasting |
UCL Support:
The Translational Research Group (TRG) have extensive knowledge of the cell and gene therapy landscape, and can provide advice on early considerations such as intended use and competitors etc, to guide development, as well as longer-term considerations for adoption i.e. help you produce and develop your TPP.
 UNMET CLINICAL NEED:
UNMET CLINICAL NEED:
As part of the TPP, comprehensively research and define the potential for benefit of the proposed therapeutic over current ‘standard of care’.
UCL Support:
The Translational Research Group (TRG) within the Translational Research Office, will help define the project plan to how your project successfully meets the unmet clinical, ensures costs are accurately calculated to cover the project sufficiently, identifies suitable funding streams and helps with the application. Importantly these funding streams require active project management which the TRG can provide throughout the entire project.
 INTELLECTUAL PROPERTY:
INTELLECTUAL PROPERTY:
Engage with Business experts to ascertain if you have a novel invention, ensure freedom to operate and to protect foreground/arising intellectual property e.g. vector composition.
UCL Support:
If you believe you have an innovative, patentable technology, it’s important you contact UCLB before any form of public domain disclosure.
UCLB are experts that provide confidential support for researchers at UCL, to understand the value of your invention and the best approach to protecting your intellectual property (IP), advising on strategies to commercialise your research such as through forming a spin out company or licensing to a third party.
All researchers with potentially commercialisable research results should fill out a confidential Invention Disclosure Form (IDF) and submit it to their UCLB Business Manager: http://www.uclb.com/for-researchers/do-you-believe-you-have-a-novel-invention/
ORPHAN DRUG DESIGN:
Sponsors can be granted special status, termed “orphan drug designation” (ODD) for a small molecule or biological product (i.e. drug) to treat a rare disease or condition. Such treatments have little commercial incentive but with specific eligibility criteria, ODD brings various development incentives for the novel treatment regarding regulatory support, clinical testing and marketing exclusivity for an orphan drug.
Applying for ODD requires submission of data and information by the Sponsor. In Europe, ODD applications are examined by the EMA's Committee for Orphan Medicinal Products (COMP) and in the USA the Food and Drug Administration (FDA).
UCL Support:
Requiring registration of the Sponsor address in the ODD market region, the Translational Research Group (TRG) within the UCL Translational Research Office (TRO), have supported the successful ODD of (two) viral gene therapies.
 PATIENT & PUBLIC INVOLVEMENT (PPI)
PATIENT & PUBLIC INVOLVEMENT (PPI)
Early PPI engagement will help raise awareness of the therapeutic during development, provide opportunities for PPI to shape the development programme, potentially facilitate patient recruitment for subsequent clinical evaluation and support financial investment.
Important PPI considerations:
- Understanding the patient and clinician needs – Does your TPP meet all of the essential requirements expressed by frontline clinical care providers, patient and public contributors (PPI)?
- Research is carried out ‘with’ or ‘by’ members of the public or potential patients etc. – Advisory members of a project steering group
Further information on PPI can be found via INVOLVE: www.invo.org.uk/
UCL Support:
The Translational Research Group (TRG) can help with the timely identification of future needs or opportunities for diversifying, as well as advising on appropriate patient groups and charities to consider.
 Close
Close

