Researchers discover new compound with potential for treating nerve damage
24 May 2023
Research led by UCL, in partnership with the MRC Laboratory of Molecular Biology and AstraZeneca, has identified a new chemical compound that can stimulate nerve regeneration after injury, as well as protect cardiac tissue from the sort of damage seen in heart attack.
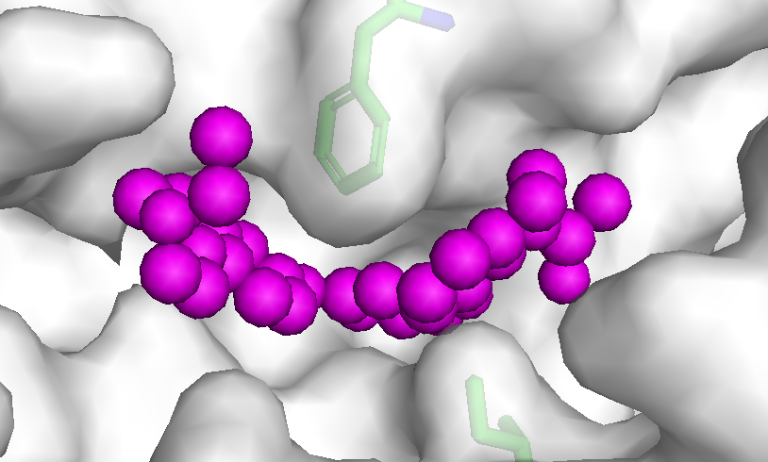
The new compound – which activates the PI 3-kinase (PI3K) signalling pathway – could lead to this class of drugs and therapies being used for a wider range of conditions.
The UCL project team, led by Professor Bart Vanhaesebroeck (UCL Cancer Institute) and Dr Richard Angell (Drug Discovery Group, Translational Research Office), in a collaboration with the MRC Laboratory of Molecular Biology (LMB) in Cambridge and AstraZeneca, report their findings in the journal Nature. They are now aiming to explore the potential of developing new treatments for peripheral nerve damage, for example sustained in serious trauma.
Finding the biological balance
Professor Vanhaesebroeck is a world-leading expert on kinases – a family of enzymes that play a pivotal role in cell signalling, influencing all manner of biological activities such as immunity, inflammation and cell growth. Many cancer cells express activated kinases, which drive malignancy, so the development of ‘inhibitor drugs’ has emerged as a way to counter this.
Over a career spanning more than 25 years, Professor Vanhaesebroeck has researched and developed some of these anti-cancer kinase inhibitors, with a particular focus on the PI3K family. However, he has long seen the potential in activating kinases to treat diseases other than cancer.
“Cancer cells are clever and are very good at exploiting the cell’s machinery to propagate and grow. This has clear negative consequences when this spirals out of control. But in some instances, we actually want to push things a bit and activate these kinases for a short time, for example after tissue damage or injury. Like everything in life, there’s a balance to be had, between inhibition and activation.”
Collaborate to innovate
Attempting to find molecular activators is not as straightforward as it is for inhibitors, due to the availability and complexity of the access sites on kinases. Professor Vanhaesebroeck therefore sought to assemble a multidisciplinary team of collaborators willing to provide funding and resources for what was seen as an ambitious project.
“Some people told me that this couldn’t be done and that it was unrealistic for even attempting it, but luckily I found people who believed in this type of research, which I’m very grateful for, because there was no guarantee of success.”
A key figure in this effort was Richard Angell (formerly head of UCL’s Drug Discovery Group, TRO) who’s team provided the critical chemistry knowledge, screening assays and contacts to crystallize Professor Vanhaesebroeck’s idea into a credible plan of action. Dr Roger Williams from the MRC-LMB in Cambridge meanwhile brought his unrivalled expertise on the structure of PI3K.
An important step in the overall success of this project has been the ongoing collaboration with AstraZeneca, the global biopharmaceutical company to utilise their extensive library of chemically diverse compounds. This collaboration initiated by Richard Angell was coordinated by Dave Smith and executed under the umbrella of an ‘Open Innovation’ programme, which sees AstraZeneca collaborating with academics that have innovative ideas to advance drug discovery and development.
Mike Snowden, Senior Vice President, Discovery Sciences, AstraZeneca strongly believes that scientific innovation and collaboration go hand-in-hand. “Our Open Innovation programme aims to create an open research environment that connects our expertise and technologies with the innovative and ambitious research ideas of collaborators like UCL and MRC-LMB, with the aim of discovering novel treatments for diseases with unmet need.”
The early work was supported by a ‘High Impact Initiative’ from the National Institute for Health and Care Research (NIHR) channelled through the UCLH/UCL Biomedical Research Centre. Additional and later funding was from the Medical Research Council, the Wellcome Trust, the British Heart Foundation, the European Union’s Horizon 2020 research and innovation programme, the UK Biotechnology and Biological Sciences Research Council, Cancer Research UK, the Rosetrees Trust and some additional funders.
Professor Vanhaesebroeck also recruited various groups at UCL as key collaborators, including from the Hatter Cardiovascular Institute, the Centre for Nerve Engineering at the UCL School of Pharmacy, the Wolfson Institute for Biomedical Research and the Department of Structural and Molecular Biology.
A potent protector and regenerator
Using high throughput screening methods to sift through the AstraZeneca compound library, the project team found a PI3K-activating hit, which they further optimised to a compound dubbed 1938, which reliably activated PI3Kα.
The next step was to assess the biological activity of 1938 through experiments on cardiac tissue and nerve cells.
During a heart attack, blood flow to cardiac tissue becomes restricted, starving cells of oxygen and potentially killing them if not restored. Re-perfusion methods, such as inserting a stent into vessels, is the first line of action, but paradoxically the flood of oxygen can damage tissue in the process of saving it.
Using a well-established experimental rodent model, UCL researchers at the Hatter Cardiovascular Institute, led by Professors Sean Davidson and Derek Yellon, found that administering the 1938 compound during the first 15 minutes of reperfusion of the infarcted heart, provided substantial tissue protection. This was shown by increased tissue survival and reduced infarct size – the area of dead tissue or necrosis caused by inadequate blood supply.
Next, researchers from regenerative medicine led by Professor James Phillips (UCL School of Pharmacy) added the 1938 compound to cultured nerve cells. They found that it significantly increased neurite outgrowth, which is a process where regenerating neurons make new projections as they grow in response to stimulus and cues.
Finally, they tested a rat model of sciatic nerve injury, delivering the 1938 compound via a single injection into the injured nerve and via a minipump implanted next to the nerve for continuous delivery. They found that 1938 treatment resulted in impressive recovery, shown by increased return of activity of the tibialis anterior muscle in the hind leg.
For Professor Vanhaesebroeck this was perhaps the most exciting and promising finding.
“There’s a huge unmet need there, because there are no current medicines that accelerate nerve regeneration, and potentially millions of people around the world could benefit.
Given the positive findings, the group is now working to develop potential new therapies for peripheral nerve damage, for example of the type sustained in serious hand injuries.
Professor Vanhaesebroeck concluded: “This is a prime example of interdisciplinary research, in which people with expertise ranging from basic science, drug development and clinical studies join forces around an innovative idea, whilst also crossing boundaries between academia and industry. 'Blue sky’ research of this kind is difficult to get funding for in a world of increasing specialisation, but hopefully this project can provide something of a model for future ambitious research.”
Further information
- Research paper: A small-molecule PI3Kα activator for cardioprotection and neuroregeneration. Nature
- Professor Bart Vanhaesebroeck’s academic profile
- Professor James Phillips’ academic profile
- Hatter Cardiovascular Institute at UCL
- UCL Cancer Institute
- UCL School of Pharmacy
- UCL Translational Research Office
- Main image: 1938 P13K activator bound to P13Kalpha
- UCL media contact: Dr Matt Midgley m.midgley@ucl.ac.uk
 Close
Close

