New research tool set to transform the way preclinical animal studies are conducted
12 February 2021
UCL-led study suggests the Clinical Relevance Assessment of Animal preclinical research (RAA) tool will help scientists and funders validate preclinical animal research prior to human clinical trials and help prevent unnecessary animal testing.
Preclinical research is used to find out whether a drug, procedure or treatment is likely to be of therapeutic benefit to humans. This type of experimental testing is often carried out using model organisms such as mice, flies, worms, and other animals. However only small proportion (around 1%) of this animal preclinical work results in clinical benefit to humans. Possible reasons for this lack of translation include the design, conduct, and reporting of preclinical studies.
To try and address this problem, Kurinchi Gurusamy - Professor of Evidence-based Medicine and Surgery at UCL, and lead author of the study - with an international team of researchers and clinicians, began to investigate whether there was a way to evaluate and refine preclinical trials in order to get more useful results.
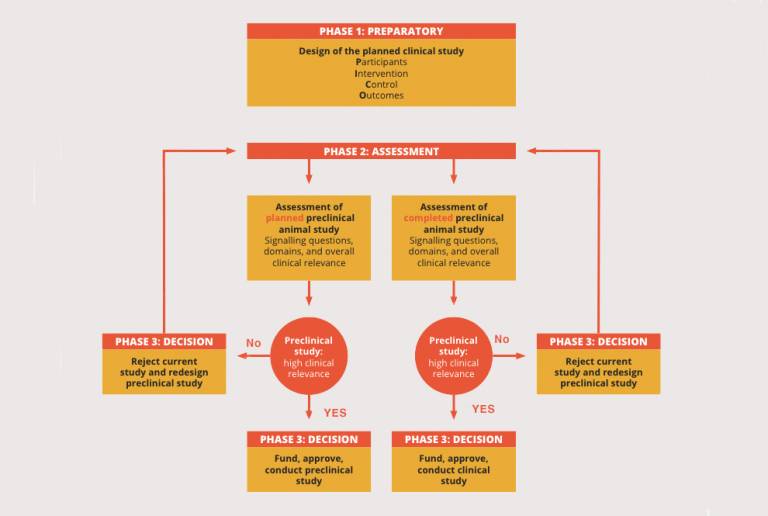
“There is currently no formal method to assess the clinical relevance of preclinical research. What we wanted to do was develop a framework to investigate the likelihood that results from an animal-based preclinical study would be of clinical benefit to human diseases. For example, if a study is poorly designed, or is using an animal model that isn’t optimal, then the results are unlikely to be applicable to human trials going forward,” explains Prof Gurusamy.
Writing in the journal PeerJ, the research team report how they reviewed current best practice guidelines for the design, conduct, and reporting of preclinical research to develop a framework to evaluate preclinical studies.
“We identified eight components (or ‘domains’) that form the basis of critically evaluating preclinical research - for example, risk of bias, reproducibility, experimental design – and structured a series of questions around these domains to form the framework. This was then reviewed and refined by a panel of medical specialists,” says Prof Gurusamy.
“The tool is currently being piloted and we think it’s likely to generate lot of academic discussions. One of the main benefits of using it will be an increased efficiency – and potential reduction - in animal studies, which we see as a very positive outcome. We hope that it will be used by funders of animal research, agencies and regulatory bodies to approve drugs and/or medical devices for clinical trial or market use.”
Further information
- Research paper: Clinical relevance assessment of animal preclinical research (RAA) tool: development and explanation. PeerJ
- Prof Kurinchi Gurusamy academic profile
- Animal research at UCL
 Close
Close

