# Recommend contact with QSCTC to get an overveiw of the process.
# Early contact with JRO
- UCL first contact questionnaire even before grant attained for UCL sponsorship (for UCL)
# Confidential disclosure agreement (CDA) for UCL studies is done via worktribe, and for UCLH via JRO.
# pre-award - worktribe tips (UCL):
- start working on application no less that 4-6 weeks prior to due date
- support for costs need to uploaded
- application needs to be uploaded
- need to subit full completed worktribe to pre-award atleast 5 days prior to grand deadline for their checks
- link for further information on costing via worktribe: https://www.ucl.ac.uk/clinical-trials-and-methodology/intranet/finance/research-costing
standard process:

- How to make a worktribe project: https://www.ucl.ac.uk/research-innovation-services/training-and-resources/worktribe-support/how-guides
# Cost completely and comprehensively.
- Don’t forget:
- Randomisation
- Patient travel reimbursement (also an important ethical issue – not to exclude subgroups of patients)
- Statistician
- MHRA cost
# IRAS form and documents
- Don’t forget patient 24hr contact card
- Invitation letters
- ALL patient facing forms/documents should be submitted
- It is a major amendment to change patient facing documents so worth doing right the first time
# Trial Steering Committee (TSC) tips
- Key Roles:
- assessing progress accoring to protocol and Sponsor timeline/milestones
- considering recommendations from the Data Monitoring Commitee; assessing patient safety and Good Clinical Practice
- assessing and advising on any major decisions e.g. continuation of trial, ammendments
- assessing any new or external information to decide if any action needs to be taken
- manage any data access requests
- oversee any complaints
- feedback to sponsor/funder and Trial Management Group
- frequency of meeting and number of members is proportionate to risk fo the clinical trial
- generally 50% independent members
BRIEF OVERVIEW OF PROCESS:
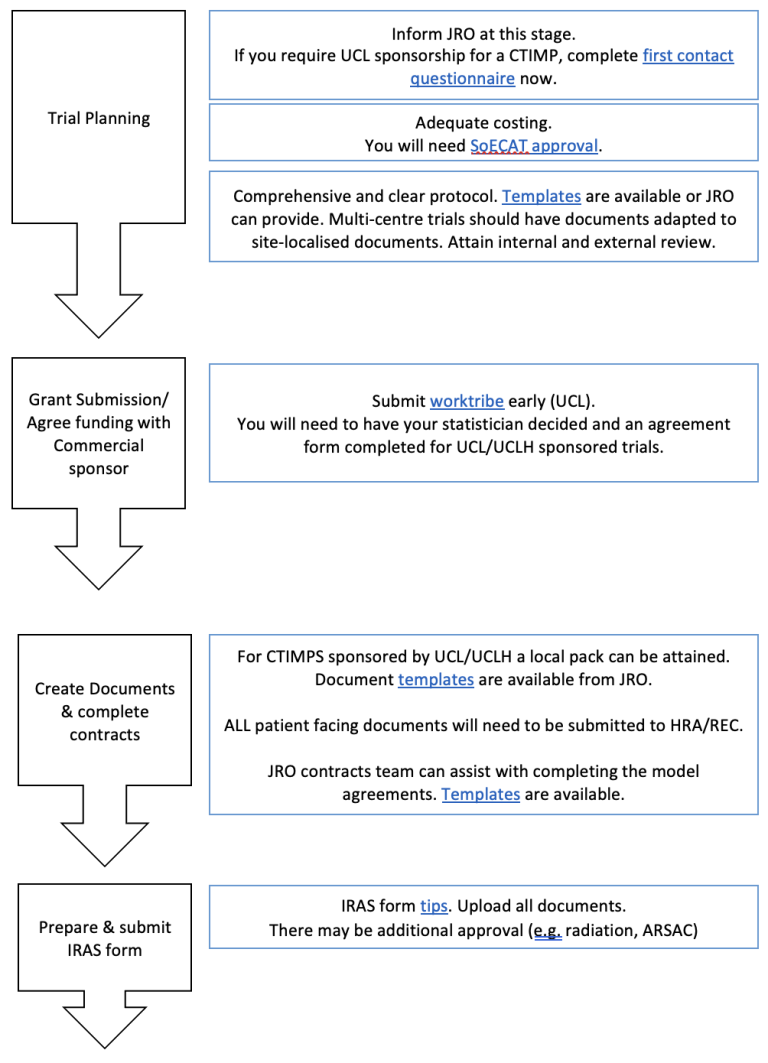
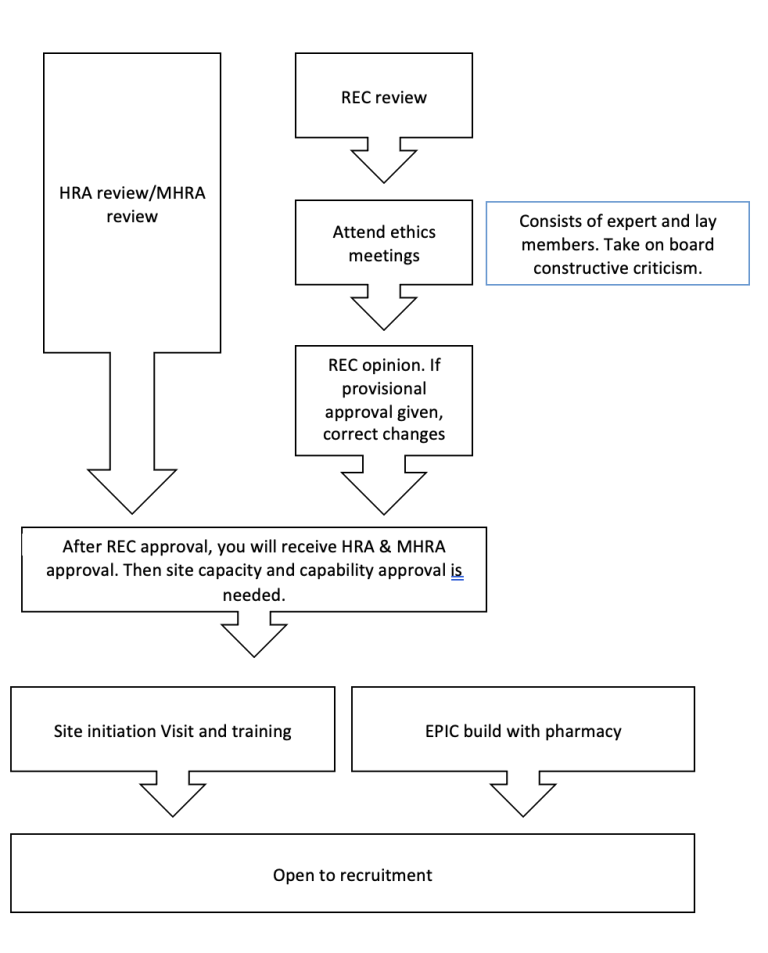
Resources of PIs:
NIHR trials roadmap:
 Close
Close

