# Contact the QSCTC to meet early to discuss the flow and approach to clinical trials.
# Early contact with JRO is adviced.
# The first contact questionnaire can be completed even before the grant is attained if sponsorship is needed.
# Cost completely and comprehensively. This is particularly important. Costing early is important to assist with negotiations.
Don’t forget to cost for:
- Randomisation
- Patient travel reimbursement (also an important ethical issue – not to exclude subgroups of patients)
- Statistician
- MHRA costs
BRIEF OVERVIEW OF THE PROCESS (for a site PI):
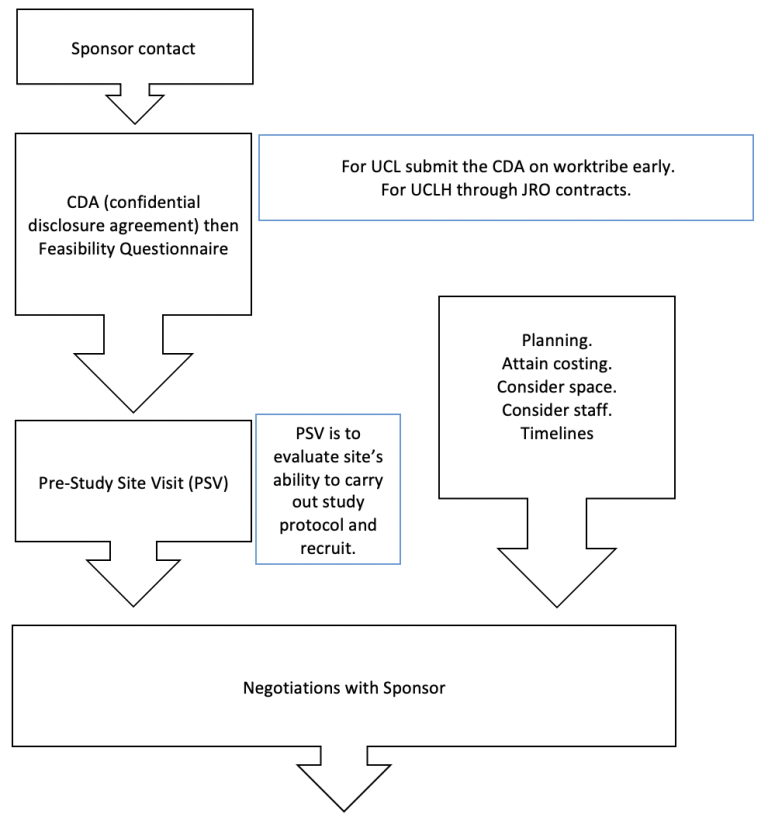
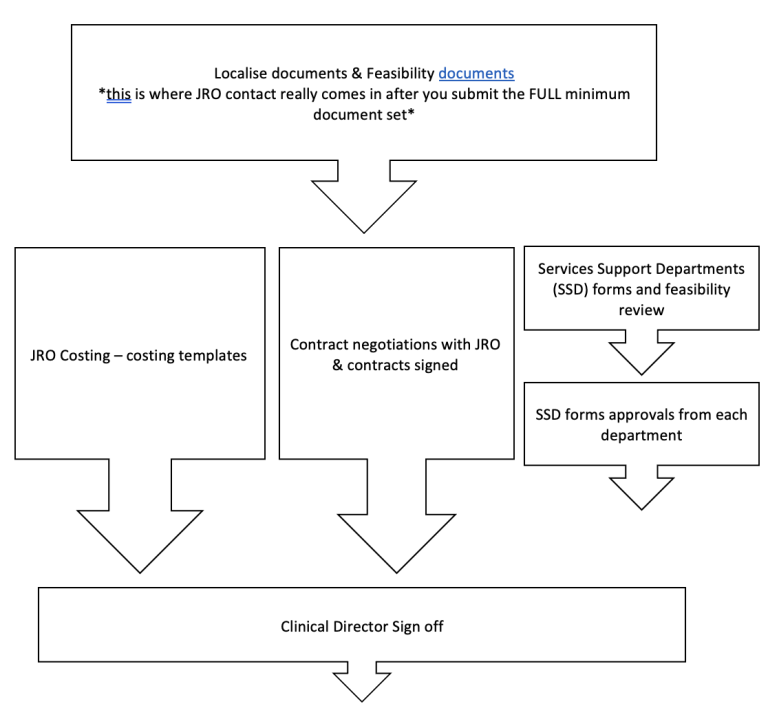
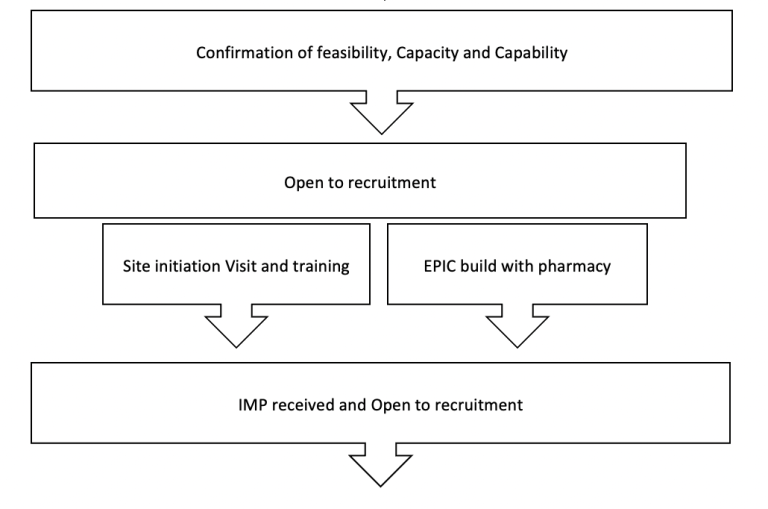
Resources for PIS:
NIHR roadmap for clinical trials:
 Close
Close

