World Alzheimer's Day: How UCL academics are spearheading the fight against Alzheimer’s disease
21 September 2023
Ten months ago, UCL’s Professor Sir John Hardy hailed the Lecanemab trial as the “beginning of the end” for Alzheimer’s disease, but this was just the start of a series of exciting developments – with UCL experts at the forefront.

It is estimated that there are currently 944,000 people living with dementia in the UK and 52% of the UK public – 34.5 million – know someone who has been diagnosed with a form of the disease. Over 60% of people living with the condition are thought to have Alzheimer’s disease.
Dementia is the nation’s biggest killer and has been the leading cause of death in women since 2011. However, there is currently no cure.
UCL is determined to tackle this growing crisis with one of the world’s largest neuroscience communities, access to a patient population of more than six million and the construction of a new neuroscience facility at 256 Grays Inn Road, enabled by a visionary community of partners and philanthropic funders.
And, over the past year, the work of the university’s talented researchers and clinicians has helped see the success of trials involving drugs such as Lecanemab and ALN-APP - showing that it is possible to slow the rate of cognitive decline in people with Alzheimer’s disease and giving hope to millions across the globe.
Where it started
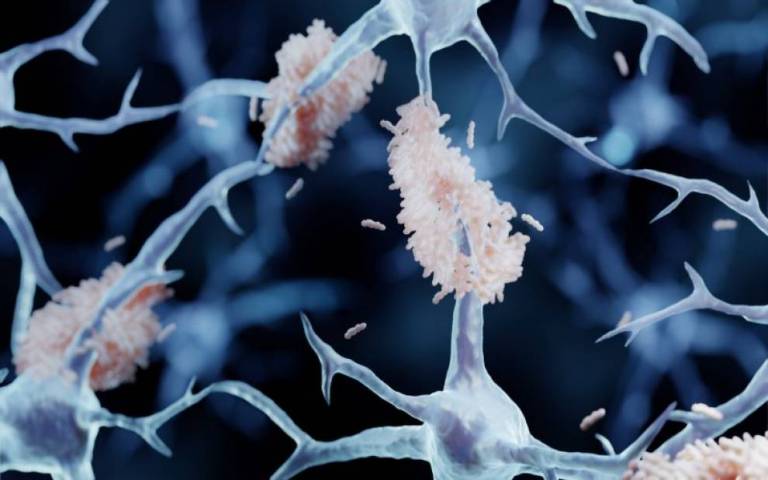
The results of the Lecanemab trial were published in November last year. It was found to slow down progression and reduce memory decline by targeting beta amyloid – a plaque that builds up in the brains of people with Alzheimer’s disease.
Professor Sir John Hardy (UCL Queen Square Institute of Neurology) was the first to identify the role of amyloid in Alzheimer’s disease 30 years ago, after he began working with Carol Jennings and her family.
Carol reached out to Professor Hardy and Professor Martin Rossor (UCL Queen Square Institute of Neurology) in the mid-1980s, after her father, his sister and brother, all developed symptoms and were diagnosed with Alzheimer’s in their 50s.
Intrigued, Professor Hardy embarked on research to determine whether there were any genetic differences between those who had Alzheimer’s and those who didn’t.
And, five years later, his team discovered a mutation to the amyloid precursor protein (APP) gene, which creates the amyloid plaques that form in the brain during Alzheimer’s disease – causing them to become overactive and signalling for ongoing inflammation, which can disrupt normal processes in the brain.
As a result of the findings, in 1992, Professor Hardy and his colleague, Professor David Allsop, published the amyloid cascade hypothesis, which helped explain:
- The brain appearing smaller due to the death of brain cells
- The build-up of amyloid protein
- Tangles with tau (a toxic protein that causes neuronal death)
It was this theory that helped pave the way for the development of Lacanemab, which provided a “modest” yet “historic” breakthrough for Alzheimer’s treatment.
The drug – developed by companies Eisai and Biogen - was shown to slow the rate of cognitive decline in people with Alzheimer’s disease.
Professor Hardy said: “This trial is an important first step, and I truly believe it represents the beginning of the end. The amyloid theory has been around for 30 years so this has been a long time coming. “It’s fantastic to receive this confirmation that we’ve been on the right track all along, as these results convincingly demonstrate, for the first time, the link between removing amyloid and slowing the progress of Alzheimer’s disease.”
And, in July, the drug was fully approved by the US Food and Drug Administration (FDA) as a treatment for early Alzheimer’s disease.
However, Lecanemab wasn’t the only drug to grab headlines in 2023.
A second turning point
In July, a new drug, Donanemab, was found to slow cognitive decline in Alzheimer’s disease by around a third, in a global trial involving 1,736 people aged 60 to 85 with early-stage Alzheimer’s.
The drug, made by Eli Lilly, works in the same way as Lecanemab, and helps in the early stages of the disease by clearing amyloid buildup in the brains of people with Alzheimer’s disease.
One American woman on the drug trial claimed that her condition improved so much that she was even able to drive and read again.
Professor Hardy and Dr Cath Mummery (both UCL Queen Square Institute of Neurology) were at the Alzheimer’s Association International Conference (AAIC) when researchers announced the findings to a room of cheering scientists and clinicians from around the world.
No magic bullets
While they admit that the results were “great news”, both Professor Hardy and Dr Mummery wanted to make clear that there were some “important caveats”. For example, it is unclear of the effect of the drug after 18 months and claims about the drug reducing disease progression by 60% were misleading.

Writing in the Daily Mail, they said: “On average, the rate of mental decline slowed by around 35% - reduced to 22% once patients with more advanced disease were taken into account.”
Additionally, both Donanemab and Lecanemab come with risks. For example, brain swelling was a common side-effect in up to a third of patients in the Donanemab trial. And while this was resolved in most cases, three volunteers died as a result.
Dr Mummery and Professor Hardy, said: “These powerful drugs carry significant risks.”
They added: “If it were given in the UK, patients would need to be carefully monitored.”
Another drug, Aducanumab, was also rejected earlier this year by European regulators over safety concerns and a lack of evidence that it was effective enough for patients.
‘Exciting’ new techniques
Whilst both Lecanemab and Donanemab focus on getting rid of amyloid proteins and need to be administered around every two to four weeks, Dr Cath Mummery is leading the UK arm of a global trial focused on preventing the amyloid protein from being produced in the first place – with effects that could last up to a year.
The ALN-APP trial started in early 2023, it is currently recruiting and so far involves 20 people, including eight in the UK.

Interim results of a phase one trial published at a medical conference in July, suggest that a single dose of the treatment reduces levels of amyloid precursor protein (APP) by up to 80%. APP is the precursor to amyloid that can build up in the brain.
The results were also found to be long lasting with tests showing that levels were still on average 65% lower six months later.
It is hoped that, if given early enough, the treatment could stop patients developing symptoms of Alzheimer’s by ‘silencing’ the gene that helps cause the disease.
Dr Mummery said: “We got the first patients in the UK into the trial. The trial involves giving the interference RNA drug to reduce the amount of protein being produced. The drug uses the cell’s own machinery to hijack the normal cell process that regulates translation of a gene into a protein. And what’s really cool is that it catalyses the normal mechanism, and so the effect of one dose can last a really long time.”
Interference RNA treatments have been approved for use since 2019 for several other diseases, including amyloidosis (where a toxic protein attacks organs in the body), acute hepatic porphyria (a genetic condition affection the nervous system) and primary hyperoxaluria type 1 (a condition that causes severe kidney stones). However, this is the first time that it has been tried in the brain for any disease.
The treatment is given via an injection in the lower back, directly into the cerebrospinal fluid surrounding the spinal cord – enabling it to reach the brain easily. The initial results show that the drug is safe.
The impact
One of the eight UK participants to be involved in the trial is Anne Warburton, 68.
Anne began repeating herself shortly before her 60th birthday and has now been diagnosed with “mild to moderate” Alzheimer’s.
Although she is still able to enjoy hobbies such as gardening, socialising and spending time with family and friends, her symptoms are gradually getting worse.
Speaking in the Sunday Times, her husband Peter, 69, said: “It means forgetting appointments, forgetting things she promised to do, misplacing items on a daily basis.”
The couple don’t know whether Anne was one of the 14 participants to receive the active drug. However, in the next multiple-dose stage of the trial, all participants will receive the genuine treatment.
Peter said: “When we decided to participate it was made very clear we were giving a gift to the medical and scientific community. “There should be no expectation that we would receive anything personally from this. But obviously we are only human, we hope this drug will give Anne a better cognitive outcome.”
The future
Now the team are starting the second part of the stage 1 trial before embarking on a larger phase 2 trial, where they hope to recruit enough people to show a slowing in cognitive decline alongside evidence of reduced production of amyloid and slowed disease.
Although there is a long way to go before the treatment is available on the NHS, Dr Mummery believes that, in the future, this kind of treatment could be effective in “helping to prevent disease rather than slow it down” – something she describes as being “really exciting”.
The method could also extend beyond Alzheimer’s to treating other genetic and non-genetic diseases involving amyloid in the future, including Cerebral amyloid angiopathy (CAA) which can cause brain swelling and bleeds, and even Down syndrome.
Dr Mummery says: “I’ve been involved in genetic therapies for eight years now. For me, conducting trials is a privilege: firstly, any trial gives the opportunity to give someone with this disease some control back of their life; being part of the team doing the trial is super-exciting regardless of at the precise trial – and that’s really why I’m in the business of conducting trials.
“Regarding this trial in particular, I think we are starting to get much more intelligent at looking at how to target diseases. We are starting to use precision medicine rather than a blunderbuss, we are trying to precisely change what is happening and, to me, that makes an awful lot of sense in medicine.
“Being at the cutting edge of something that has never been done before is brilliant. I wouldn’t do any other job, I love it and feel honoured to be able to work in this area.”
Links
- Find out more about how UCL is tackling the global challenge of dementia
- Professor John Hardy's academic profile
- Dr Cath Mummery's academic profile
- UCL Queen Square Institute of Neurology
- UCL Brain Sciences
- UCL Giving: Neuroscience
Images
- Doctor diagnose and checking brain testing result with computer and AI, robotic on virtual interface in laboratory, innovative technology in science and medicine. Credit: ipopba on iStock
- Amyloid plaques are misfolded protein aggregates between neurons - Alzheimer's disease illustration. Credit: Artur Plawgo on iStock
- Professor Sir John Hardy
- Dr Cath Mummery
- Closeup of an old caucasian man and an old caucasian woman sitting in a couch holding hands with affection. Credit: nito100 on iStock
 Close
Close


