New research uses a human stem cell model to uncover disease mechanisms in dementia
6 November 2023
Research from Race Against Dementia Fellow Dr Christy Hung and Professor Rickie Patani shows that the increase of a particular type of tau protein—called the 4R isoform—drives degeneration of nerve cells, thereby causing Frontotemporal dementia (FTD).
Frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) are two untreatable neurodegenerative diseases that exist on a clinical, genetic, and pathological spectrum.
The VCP gene is of significant relevance, directly implicated in both FTD and ALS. This study investigates the effects of VCP mutations on the cellular homeostasis of hiPSC-derived cortical neurons, with a focus on endo-lysosomal biology and tau pathology.
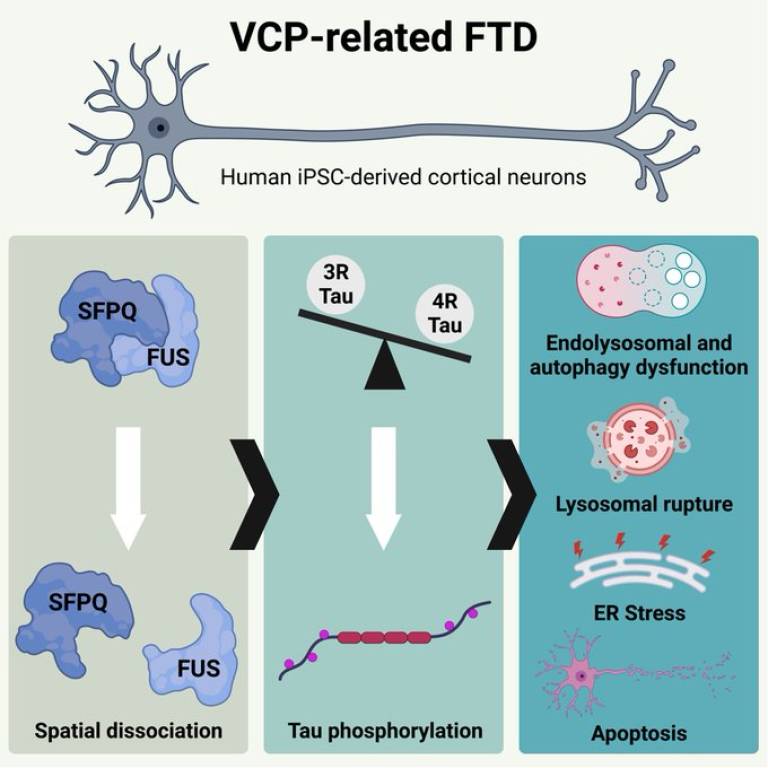
Dr. Christy Hung, a Race Against Dementia Fellow and Alzheimer's Research UK Senior Fellow at UCL and the Francis Crick Institute, led this research. Her work is generously supported by Race Against Dementia, a charity founded by motorsport legend Sir Jackie Stewart OBE, which leverages the high-performance mindset of Formula 1 to accelerate progress toward finding a cure for dementia. Together with Professor Rickie Patani (Professor of Human Stem Cells and Regenerative Neurology, UCL Queen Square Institute of Neurology and The Francis Crick Institute, and a consultant neurologist at The National Hospital for Neurology and Neurosurgery, UCLH), they have discovered that VCP mutations cause an abnormal accumulation of enlarged endo-lysosomes, accompanied with impaired interaction between nuclear FUS and SFPQ in human cortical neurons.
The spatial dissociation of intra-nuclear FUS and SFPQ correlates with alternative splicing of the MAPT pre-mRNA and increased tau phosphorylation. Importantly, this study reveals that an increase in the 4R-tau isoform is sufficient to drive toxic changes in healthy human cortical neurons, including tau hyperphosphorylation, endolysosomal dysfunction, lysosomal membrane rupture, endoplasmic reticulum stress, and apoptosis. Their data suggests that endolysosomal dysfunction could represent a convergent pathogenic ‘design principle’ shared by both FTD and ALS.
“A tidy brain is a healthy brain. Dysfunction of the endolysosome pathway is emerging as an important pathogenic process shared by multiple neurodegenerative diseases, such as Alzheimer's (AD), Parkinson's (PD), and frontotemporal dementia/amyotrophic lateral sclerosis (FTD/ALS). Our study reveals that by increasing 4R tau through antisense oligonucleotide technology, we can induce harmful changes in healthy human neurons. These changes include tau hyperphosphorylation, endolysosomal dysfunction, lysosomal membrane rupture, endoplasmic reticulum stress, and apoptosis, mirroring the behaviour of VCP-mutant neurons. This discovery opens the possibility of therapeutically targeting 4R tau to alleviate endolysosomal dysfunction.” – Dr Christy Hung
Links
- Christy Hung, Rickie Patani, Elevated 4R tau contributes to endolysosomal dysfunction and neurodegeneration in VCP-related frontotemporal dementia, Brain, 2023;, awad370, https://doi.org/10.1093/brain/awad370
- Professor Rickie Patani's academic profile
- Dr Christy Hung's academic profile
- Dr Christy Hung’s Race Against dementia profile
 Close
Close

