Investment in new Patani Lab research to unravel the impact of DNA damage in Motor Neuron Disease
24 November 2023
We are pleased to announce that My Name’5 Doddie Foundation recently welcomed Professor Rickie Patani of the Francis Crick Institute and UCL to their growing list of Foundation-funded researchers, working to help them achieve their vision of a world free of MND.

In this important study, Professor Patani (Professor of Human Stem Cells and Regenerative Neurology at UCL Queen Square Institute of Neurology and The Francis Crick Institute, and consultant neurologist at The National Hospital for Neurology and Neurosurgery, UCLH) and his co-investigators, Dr Giulia Tyzack (Senior Research Fellow, Department of Neuromuscular Diseases, UCL Queen Square Institute of Neurology) and Professor Simon Boulton, aim to build on emerging data that suggests that DNA damage may contribute to the development and/or progression of MND. If the team finds that DNA damage is an important contributor to MND biology, they hope to unravel the biological process at play and identify new targets for future treatments
Errors in DNA repair cause genomic instability and cell death
Many different factors can cause DNA damage in all cells in the body, and our cells have several mechanisms to repair the damage. Occasionally however, errors occur in the repair process leading to mutations in our DNA. When many errors occur, the accumulation of mutations and resulting “genomic instability” can lead to widespread dysfunction within the cell, and ultimately cell death.
Increased DNA damage in motor neurons has been observed in both cellular models of MND and also in people living with familial forms of the disease. Professor Patani’s group has also demonstrated an enhanced DNA damage response as a “signature” of disease in both sporadic and familial forms. This evidence has led to the hypothesis that DNA damage could be a major contributor to the development and progression of MND.
Investigating the link between DNA damage, RNA processing and MND
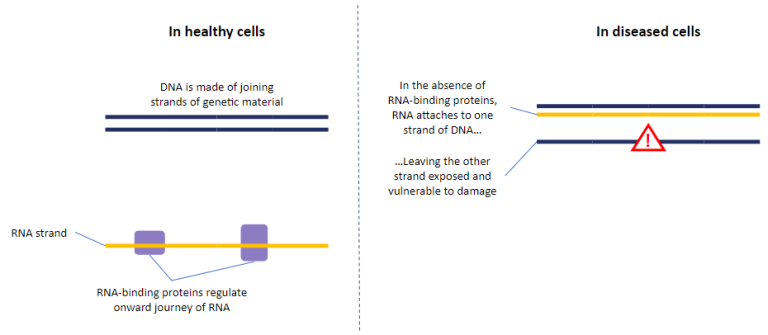
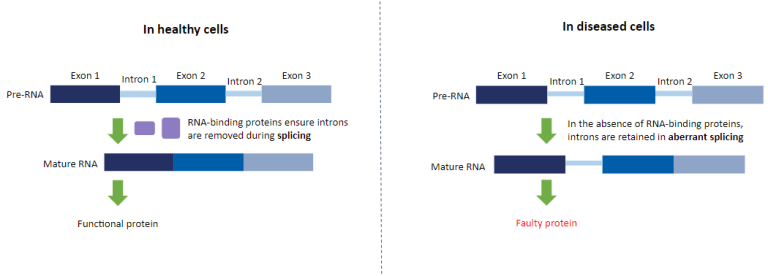
One of the most common pathological hallmarks of MND is the mislocalisation and loss of function of the RNA-Binding Protein, TDP-43, affecting approximately 97% of people living with the disease. RNA-Binding Proteins like TDP-43 regulate which genes the cell “turns on and off”, and the function of many of these proteins is lost in MND.
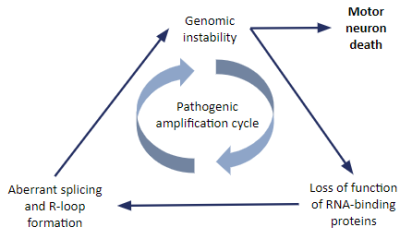
Dr Tyzack together with Professor Patani and Professor Boulton will explore the hypothesis that the loss of function of RNA-binding proteins in MND causes genomic instability through two processes, called R-loop formation and aberrant splicing.
The resulting genomic instability caused by these processes could lead to further loss of function of RNA-binding proteins, leading to a pathogenic amplification cycle which ultimately leads to the death of motor neurons.
How the team will test their hypothesis
First, the team will use DNA and RNA sequencing of induced pluripotent stem cells (iPSCs - cells taken from the skin of people living with MND and reprogrammed in the lab into motor neurons) to understand the characteristics of DNA damage that occurs in motor neurons at different timepoints in their development.
Fluorescent imaging and molecular biology techniques will then determine the presence of R-loops at these different timepoints. The data will be used to establish if there is a crosstalk between R-loop formation, aberrant splicing and DNA damage in MND, and the order in which each event happens.
The team will then validate these findings in animal models of MND and post mortem spinal cord tissue from people who have died from MND and donated their tissue to research. This work could pave the way to explore new therapies to prevent genomic instability associated with the loss of RNA-binding proteins, which could be effective in people living with both sporadic and familial forms of MND.
Dr Giulia Tyzack, Senior Research Fellow leading this study, said “We are very grateful to My Name’5 Doddie Foundation for funding the next stage of our research. We are indeed very excited to start working on this project which will allow us to unravel the relationship between fundamental disease mechanisms underlying MND. We believe that combining our lab’s knowledge of MND models and RNA biology together with Prof Boulton’s world-leading expertise in the DNA damage field, we are optimally poised to make impactful discoveries for MND therapy”.
Jessica Lee, Director of Research at My Name’5 Doddie Foundation, said “We are committed to funding research that will accelerate the development of new treatments for MND; a devastating condition which currently has no effective treatments. The exciting and potentially ground-breaking nature of this project is reflected in the significant investment we are making. When we launched our new research strategy, Catalysing a Cure, this kind of project, with the potential to open up new therapeutic avenues, was exactly what we had in mind.”.
We’re hugely grateful to our supporters in Hong Kong, whose generous donations are funding this exciting work that could lead to the identification of new targets for treating MND. We will work closely with the research team to ensure that each milestone throughout the project is met before releasing further funds, ensuring that every pound is spent on progressing only the most promising results. We’d also like to acknowledge the MND Association which is providing a further £50k towards the project.
Links
- My Name’5 Doddie Foundation
- Professor Patani's academic profile
- Dr Giulia Tyzack's academic profile
- Patani Lab
Source
 Close
Close