Donor motor neurons could restore muscle function in amyotrophic lateral sclerosis (ALS)
23 August 2023
Scientists led by UCL Queen Square Institute of Neurology have detailed how a combination of grafted replacement motor neurons and optical nerve stimulation – using light to activate neurons – can improve muscle function in a highly aggressive mouse model of ALS.
Their research in mice, published as an eLife Reviewed Preprint, is described by the editors as a fundamental study that presents convincing evidence for the restoration of muscle innervation and contractions in an advanced form of ALS. The findings could eventually pave the way for an assistive therapy that can be uniformly applied to all ALS patients.
ALS is the most common form of Motor Neurone Disease (MND) in adults. An early hallmark of ALS is the breakdown of neuromuscular junctions – the connection between the end of a motor nerve and a muscle – which causes the muscle to become denervated. This breakdown leads to muscle weakness, paralysis and ultimately a premature death. The median survival time in ALS, following initial onset of symptoms, is around 20–48 months.
“The cellular and molecular changes that underlie neuron degeneration in ALS are extremely complex, and can vary greatly between individual patients,” explains study lead and co-senior author, Dr Barney Bryson, MND Association Senior Non-Clinical Research Fellow and NIHR BRC UCL Excellence Fellow, in the Department of Neuromuscular Diseases, UCL Queen Square Institute of Neurology, London, UK. “Due to this, there are currently no therapies that can prevent the progression of symptoms in ALS patients.”
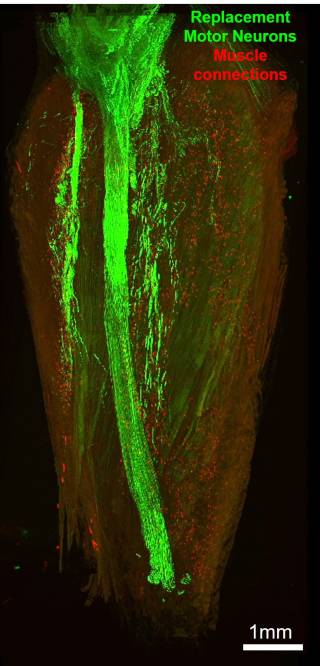
Bryson and colleagues have previously demonstrated a proof-of-concept strategy to overcome muscle denervation in a nerve injury model of muscle paralysis in mice. The strategy uses a technique called optical nerve stimulation (ONS) to stimulate grafted replacement motor neurons which have been modified to be light sensitive, using a small light-emitting diode. In the current study, the team aimed to determine whether this strategy could be adapted to reinnervate and restore muscle function in a highly aggressive model of ALS.
First, the team sought to ensure the donor healthy motor neurons could survive the grafting process and would not be attacked by the recipient’s immune system. After tests with the immunosuppressive drug tacrolimus – which is regularly used in human organ transplants – showed it was unsafe for use in the ALS mouse model, they looked for a more specific form of immunosuppression, leading them to try a type of antibody called H57-597. This treatment helped prevent graft rejection and successfully restored some of the nerve connections to the target muscles. However, the force of the muscle contractions afforded by the treatment was still relatively weak.
The formation and maintenance of neuromuscular junctions are activity-dependent processes. This means that, without regular stimulation, grafted motor neurons may survive but are unlikely to form mature neuromuscular junctions – which could explain the weak muscle contractions observed by the team. To ensure regular stimulation, they therefore used a wireless optical stimulation system in the mice to impose regular muscle contractions for 1 hour each day. After 21 days of this optical stimulation training, the mice showed a more-than 13-fold improvement in muscle contraction force.
These findings are important as they show that affected muscles in an ALS mouse model remain receptive to reinnervation by healthy engrafted motor neurons, even until the late stages of the disease.
The authors say there are still many challenges to overcome before this approach could be used to restore muscle function in ALS patients. Further studies are required to validate whether the grafting procedure would work with human motor neurons, and whether it would be sufficient to improve the patient’s quality of life. Furthermore, the approach needs to be tested in other forms of MND, especially those with a longer life expectancy, to validate the long-term effectiveness of the procedure.
“Our study demonstrates that replacement motor neurons can robustly and reliably reinnervate target muscles in an advanced model of ALS,” concludes senior author Linda Greensmith, a Professor in the Department of Neuromuscular Diseases, UCL Queen Square Institute of Neurology. “If this approach can be successfully translated to ALS patients, the redundancy of the motor neuron subtype used would mean that a single type of motor neuron could be produced to target a large number of different muscles in individual ALS patients. This in turn would lead to a more efficient and wide-scale treatment option.”
This research was funded by the Motor Neurone Disease Association, NIHR UCLH BRC, The Rosetrees Trust, The Medical Research Council and The Richard Stravitz Foundation
Links
- Bryson et al. (2023) An optogenetic cell therapy to restore control of target muscles in an aggressive mouse model of Amyotrophic Lateral Sclerosis eLife 12:RP88250. https://doi.org/10.7554/eLife.88250.1
- Dr Barney Bryson's academic profile
- Professor Linda Greensmith's academic profile
- Queen Square Motor Neuron Disease Centre, UCL Queen Square Institute of Neurology
Source: eLife
 Close
Close