MDC Publication Highlights - February-March 2020
28 April 2020
Understanding Parkinson's disease dementia: Increased brain iron deposition and White matter abnormalities, Pathological features of rapidly progressive cortico basal degeneration
Increased brain iron deposition as biomarker for increased risk of dementia in Parkinson’s disease
Dementia affects up to 50% of people with Parkinson’s disease (PD) but the severity and timing of cognitive involvement is widely different between individuals. Currently there are no good clinical tools to predict who is more likely to develop dementia or to track cognitive decline in its earliest stages. Although several proteins thought to be involved in Parkinson’s disease dementia (PDD) have been identified (such as amyloid, tau and alpha-synuclein), the biological processes leading to dementia, and in particular the reasons for the selective vulnerability of particular brain regions remain unclear. As people age, there is an accumulation of iron in the brain, often in combination with accumulation of other proteins linked to PDD (e.g., amyloid, tau). As such, it has been suggested that the detection of brain iron could be a sensitive way to identify the brain circuitry affected by the earliest processes that ultimately lead to PDD.
The current research is a collaboration between UCL researchers from the Dementia Research Centre and the Movement Disorders Centre. The researchers used a brain imaging analysis technique called quantitative susceptibility mapping (QSM) to investigate brain changes related to cognitive deterioration in PD. Unlike traditional MRI, which looks at changes in brain volume, QSM detects local variations in iron content. The study's findings showed that dementia risk can be linked to increased brain iron content in several specific brain regions (such as the parietal and frontal cortices). More importantly, the researchers found a correlation between brain iron levels and the severity of motor and cognitive symptoms in people with PD. They found that increased region-specific brain iron levels were associated with increased risk for cognitive impairment and dementia. As such, brain iron levels can be used to track cognitive changes in PD and may prove a useful tool to stratify patients for clinical trials as well as to monitor disease progression.
Thomas et al. ‘Brain iron deposition is linked with cognitive severity in Parkinson’s disease’. Journal of Neurology, Neurosurgery and Psychiatry, First published: 20 February 2020, DOI: 10.1136/jnnp-2019-322042.
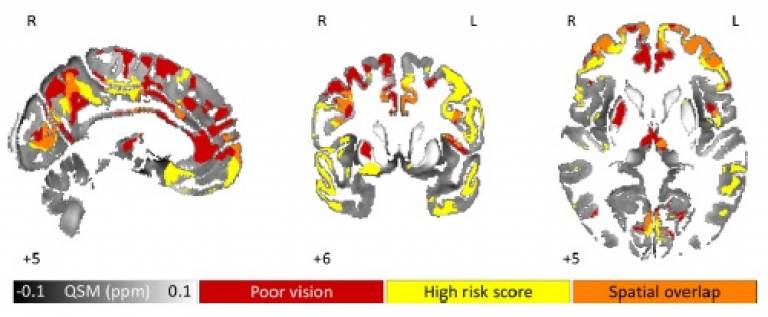
White matter abnormalities hint at mechanism underlying hallucinations and visual dysfunction in Parkinson’s
Dementia is common for individuals with Parkinson’s disease (PD) over the age of 65, but there is currently no way of determining which individuals will go on to develop dementia. However, a few symptoms have been identified as being associated with increased risk for dementia, including the occurrence of visual hallucinations and abnormalities in visual processing. Therefore, understanding the neural mechanisms of these may provide important insight into Parkinson's disease dementia.
MDC researchers, led by Dr. Rimona Weil, used a novel neuroimaging technique, called fixel-based analysis, to investigate structural brain changes associated with visual hallucinations and low visual performance in PD, focusing on changes in brain fibres (white matter). This technique allowed the researchers to estimate both the connectivity properties of different fibre populations in the brain as well as to identify and quantify any degenerative changes. Findings of this study indicated that individuals with Parkinson’s who experience visual hallucinations had large-scale structural changes in specific parts of some of the brain’s major fibre pathways (e.g., the corpus collosum, splenium, thalamic radiation), compared to people with Parkinson’s without hallucinations. People with Parkinson’s and low visual performance also had changes in white matter structure, as well as microstructural changes in fibre density, indicating a reduction in connectivity. Overall, these findings support the idea of attentional network changes as a possible mechanistic model for hallucinations in Parkinson’s, and provide insight into the early stages of cognitive changes in PD.
Zarkali et al. ‘Fiber-specific white matter reductions in Parkinson hallucinations and visual dysfunction’. Neurology, First published: 24 February 2020, DOI: 10.1212/WNL.0000000000009014.
Pathological features of rapidly progressive cortico basal degeneration
Cortico basal degeneration (CBD) is an atypical Parkinsonian disorder with a relentlessly progressive disease course, with a disease duration ranging from 5 to 7 years. However, several rare cases have been described in which the disease progressed very rapidly, with patients succumbing to the illness within 3 years or less. Rapidly progressive neurodegenerative conditions may not follow classically defined diagnostic criteria, and with several different conditions showing clinical features similar to rapidly-progressive CBD (RP-CBD), there is a significant risk of patients being clinically misdiagnosed. The current study, led by researchers at the Queen Square Brain Bank, sought to investigate the neuropathological characteristics of RP-CBD in autopsy-confirmed cases, to understand how they may differ from other types of CBD.
The researchers examined the neuropathological characteristics of six RP-CBD cases and compared them to 12 age-matched cases with typical gradually progressive CBD, who had reached the advanced clinical/end stage of the disease. Findings of this study indicated that despite shorter disease duration, RP-CBD cases had developed advanced pathological changes within ~2.5 years, similar to those usually seen in end stage CBD after an average of ~6-7 years. A major distinctive feature of RP-CBD was the predominance of tau aggregates in neurons (rather than in astrocytes), exceeding the levels usually observed in other types of CBD. These findings suggest that RP-CBD is a distinctively aggressive variant of CBD with an accelerated degenerative process, which is seen in both clinical symptoms and pathological features.
Ling et al. ‘Fulminant corticobasal degeneration: a distinct variant with predominant neuronal tau aggregates’. Acta Neuropathologica, First published: 16 January 2020, DOI: 10.1007/s00401-019-02119-4.
 Close
Close

