MDC Publication Highlights - October 2019
12 November 2019
Validation of a new Parkinson’s non-motor rating scale, The links between Parkinson’s and cardiovascular disease, Sensory attenuation provides clues to the neural mechanism underlying bradykinesia in Parkinson’s & Cognitive safety of Deep Brain Stimulation for Tourette’s.
Validation of a new Parkinson’s non-motor rating scale
Despite primarily being considered a movement disorder, individuals with Parkinson’s disease commonly experience a range of non-motor symptoms. These include, among others: anxiety, depression, cognition and sleep disturbances, urinary and gastrointestinal problems, sexual dysfunction and more. A well-established scale for measuring the impact and burden of non-motor symptoms on the quality of life of people with Parkinson’s is the Non-motor Symptoms Scale (NMSS). However, this scale has several limitations both in terms of its structure and scoring as well as in lacking assessment of more recently described non-motor symptoms. As such, a multicentre study lead by MDC researcher Prof. Anette Schrag and international collaborators investigated the validity of an updated version of the scale: The Movement Disorder Society Non-Motor Rating Scale (MDS-NMS). This updated scale contains additional domains not included in previous scales (such as impulse control and related disorders), as well as expanding on domains assessing cognition and other neuropsychiatric aspects of Parkinson’s. Four-hundred people with Parkinson’s participated in a multi-centre study evaluatng the utility and validity of the updated scale. Study participants completed an extensive battery of clinical assessments, evaluating features such as motor symptoms, non-motor symptoms, mood, quality of life, cognitive function and more. Findings of this study indicated that the new scale effectively captured a broad range of non-motor symptoms of Parkinson’s in terms of both severity and frequency of such symptoms. In addition, the scale presented with good reliability and precision, making it a suitable outcome measure to be used in clinical trials, in research and as a tool for guiding healthcare in Parkinson’s.
Chaudhuri et al. ‘The Movement Disorder Society Nonmotor Rating Scale: Initial Validation Study’. Movement Disorders, First published: 30 September 2019, DOI: 10.1002/mds.27862.
What can we learn from the links between cardiovascular disease and Parkinson’s disease?
During the search to understand the causes of Parkinson’s disease, a large array of research studies has investigated the associations between different genetic or environmental factors and PD. Many of the factors that have been found as either increasing or lowering the risk of Parkinson’s are also associated with cardiovascular disease. These observations raise questions about the nature of these associations and the likelihood of a possible common mechanisms underlying both Parkinson’s and cardiovascular disease. This article reviews the epidemiological evidence for various environmental and life-style factors which have either a similar effect of opposite effect on the risk for Parkinson’s and cardiovascular disease. The researchers discusses the cellular and metabolic processes that may underlie these associations, and explore the implications this has for patient care and future research.
Potashkin et al. ‘Understanding the Links Between Cardiovascular Disease and Parkinson’s Disease’. Movement Disorders, First published: 4 September 2019, DOI: 10.1002/mds.27836.
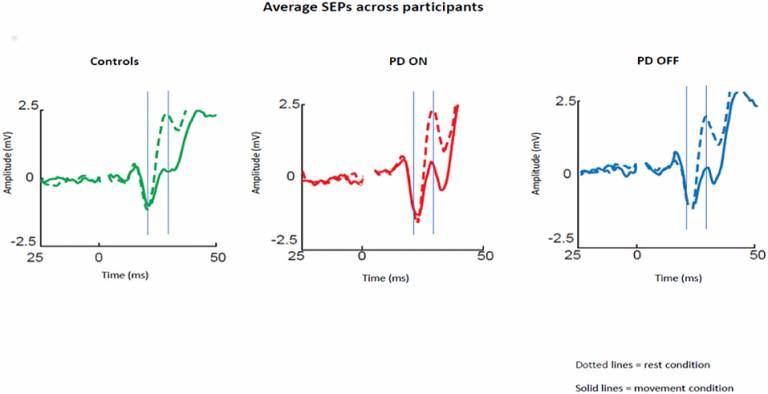
Neurophysiological mechanism potentially underlying slowed movements in Parkinson’s Disease
“Sensory attenuation” is the phenomenon whereby the brain’s response to sensory information (e.g., a sensation of touch) is reduced just before and during active movement. A recent theory hypothesized that sensory attenuation is an essential mechanism of voluntary movement. An impairment of sensory attenuation is a well-documented observation in people with Parkinson’s, but it is unclear whether it is the cause of movement initiation problems in seen Parkinson’s. The current study by MDC researcher and colleagues investigated whether the level of sensory attenuation was affected by dopaminergic medication in Parkinson’s disease and whether it could account for some of the motor symptoms observed in people with Parkinson’s. Researchers measured the brain’s response to sensory inputs by applying a small electrical stimulation to on of the nerves coming from the wrist and used EEG to measure brain activity in response to stimulation. Sensory attenuation was measured by comparing the amplitude of the brain response when stimulation was applied to the wrist at rest or just before study participants moved their thumb. This study found that, as previously reported, people with Parkinson’s who were off medication had a lower sensory attenuation compared to healthy individuals. In contrast, people with Parkinson’s who were on medication had a larger (and closer to normal) sensory attenuation. These results suggest that sensory attenuation could be the neurophysiological mechanism underlying some of the motor symptoms seen in Parkinson’s disease, primarily bradykinesia – slowness of movements and difficulty to initiate movements.
Macerollo et al. ‘Dopaminergic Modulation of Sensory Attenuation in Parkinson's Disease: Is There an Underlying Modulation of Beta Power?’. Frontiers in Neurology, First published: 18 September 2019, DOI: 10.3389/fneur.2019.01001.
Cognitive safety of Deep Brain Stimulation for Tourette’s Syndrome
Tourette’s syndrome (TS) is a neurodevelopmental disorder characterised by the presence of motor and vocal tics. For the majority of patients these tics can be treated through medications and behavioural treatments. However, for 10-20% of patients such treatment is ineffective or causes unbearable side-effects. For these patients deep brain stimulation (DBS) targeted bilaterally to a region called the anteromedial globus pallidus internus (GPiam) can be an effective alternative treatment, as demonstrated in two previous clinical trials. The current study, lead by researchers from MDC and the National Hospital for Neurology and Neurosurgery’s Functional Neurosurgery Unit, investigated the effects of GPi DBS on cognitive function in patients who have undergone surgery. Study participants completed an extensive neuropsychological battery of cognitive tests evaluating their verbal fluency, executive function, different aspects of attention and memory. Cognitive tests were one prior to the DBS surgery and then repeated at three and six months post-surgery. Outcomes of the study showed that GPiam DBS had no negative effect on cognition. This provides further support for the cognitive safety of DBS as a treatment option in TS.
Cappon et al. ‘Globus pallidal deep brain stimulation for Tourette syndrome: Effects on cognitive function’. Parkinsonism & Related Disorders, First published: 14 October 2019, DOI: 10.1016/j.parkreldis.2019.10.013.
 Close
Close

