Nature Medicine publish a new paper by Professor Spiros Denaxas and colleagues
7 October 2019
“Avoidable flaws in observational analyses: an application to statins and cancer” was published today by Nature Medicine.
Randomised trials may not provide timely answers to inform clinical, policy, and regulatory decisions. The limitations of these trials to support decision making are most evident when the goal is to evaluate long-term harms and benefits or to estimate effects in subgroups of individuals. In these cases, the analysis of observational data provides an opportunity to generate evidence to inform decisions.
The increasing availability of large healthcare databases, combined with recent computational and analytic developments, is fuelling a debate on whether observational data from electronic health records can play a role in assessing the benefit-risk of medical treatments.
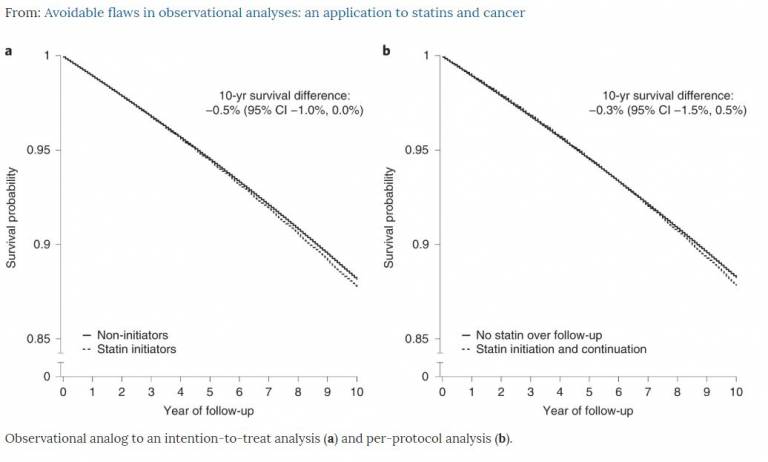
This paper illustrates that observational studies failures result from deviating from basic principles of study design rather than from a lack of randomization. The group implemented a four step approach;
- They specified the protocol of a target trial to estimate the effects of statins on cancer incidence among adults with specific low-density lipoprotein (LDL) cholesterol levels. They used a large observational database to emulate this target trial.
- They used the same observational data to replicate a previous observational study that reported a substantially lower cancer risk among statin users compared with nonusers.
- They then repeated the analysis to emulate a target trial of statin therapy and type 2 diabetes (rather than cancer) proving the generality of their approach.
The group which included the UCL IHI’s Professor Sprios Denaxas, summarised that statin therapy does not influence cancer incidence and that explicitly emulating a target trial helped reduce the discrepancies between the effect estimates from observational analyses and randomized trials. Importantly, they highlight the crucial role of factors other than randomization in explaining discrepant observational versus randomized effect estimates.
This study used primary care electronic health records accessed through the CALIBER resource and mirrored the randomized trial protocol.
To read the full paper visit the Nature Medicine website.
Paper’s full citation: Barbra A. Dickerman, Xabier García-Albéniz, Roger W. Logan, Spiros Denaxas & Miguel A. Hernán. (2019) Avoidable flaws in observational analyses: an application to statins and cancer. Nature Medicine 10;1-6 doi.org/10.1038/s41591-019-0597-x
 Close
Close

