Our work combines statistical analysis, mathematical modelling and detailed work with clinical teams, patients, families, commissioners and audit bodies to understand, improve and communicate outcomes
CORU staff involved: Christina Pagel, Sonya Crowe, Martin Utley, Ferran Espuny Pujol, Qi Huang, Julie Taylor, Luca Grieco
- CHAMPION (2019 - 2024)
CHAMPION: Congenital Heart Audit: Measuring Progress In Outcomes Nationally (2019 - 2024)

Services for Congenital Heart Disease (CHD) span a patient’s lifetime, but their quality is currently mainly measured by 30-day survival following children’s heart surgery. 30-day survival, now almost 98%, is no longer a good proxy for quality. With the number of adults with CHD now outnumbering the paediatric population, the lack of routine monitoring of adult services is particularly problematic.
Complementing and following on from the LAUNCHES project, CORU's Christina Pagel and Sonya Crowe are leading a team of clinicians, commissioners, patient representatives and academics to address several gaps in current national outcome reporting.
The project, nicknamed CHAMPION, is funded by the UK National Institute for Health Research Policy Research Programme (NIHR PRP).
Questions we will answer
We will apply to use the LAUNCHES data (see below), which links national audit data on procedures for congenital heart disease and admissions to adult and paediatric intensive care, life status from ONS and Hospital Episode Statistics (HES). We will use this data to answer the following questions:
1) What happens to adults after operations?
The percentage of adults surviving certain procedures are publicly reported, but there is no overview by hospital because there is no way to take into account the complexity of each operation. Working with clinicians, patients and charities, we will identify what outcomes capture quality of service (including survival one month after surgery) and how these can be reported fairly, developing risk adjustment methods where needed.
2) What happens in the longer-term for a person?
From both a patient and an NHS perspective, it is more relevant to understand what happens to people over the longer-term (and possibly many operations) rather than just one month after surgery. Working with clinicians, patients and charities, we will choose a range of CHD diagnoses from relatively straightforward to very complex and track people with each of these through the combined dataset, describing their:
• Long-term survival;
• How many operations and hospital stays they have;
• How many operations are outside the anticipated treatment plan.CHAMPION is a research study funded by the National Institute for Health Research. The principal investigators are Dr Sonya Crowe and Professor Christina Pagel, and the study sponsor is University College London (UCL). The study aims to improve how the quality of services for congenital heart disease (CHD) is measured and reported.
No new data will be collected as part of the study: the research will use data already collected routinely across England and Wales. Most of the data being used for CHAMPION is already held at a secure location at University College London for use in another study (called LAUNCHES) that is also being led by Dr Sonya Crowe and Professor Christina Pagel.We are applying to use data from the following sources:
1. The National Congenital Heart Disease Audit (NCHDA)
2. The Paediatric Intensive Care Audit Network (PICANet)
3. The Intensive Care National Audit and Research Centre Case Mix Programme (ICNARC-CMP)
4. Hospital Episode Statistics (HES)
5. Office of National Statistics Death Registrations (ONS)
6. The National Adult Cardiac Surgical Audit (NACSA)
7. The National Congenital Anomaly and Rare Disease Registration Service (NCARDRS)
8. An anonymised clinician derived dataset from Barts Health NHS TrustPlease note that the data we will hold will not include the patient identifiers name, address, date of birth or postcode (it will be pseudonymised).
Click to view the
with further information on the data we are collecting and why, and who to contact if you do not want information about you, or the child you care for, to be used for the CHAMPION study.Click on the links below to view the CHAMPION monitoring longer-term outcomes by diagnoses: inclusion and treatment pathway rules for the nine sentinel diagnoses.
Click on the link below to view the CHAMPION monitoring longer-term outcomes by diagnoses: mappings used to identify patients with congenital non-cardiac comorbidity and pre-term birth.
- LAUNCHES QI (2018-2023)
LAUNCHES QI: Linking audit and national datasets in congenital heart services for quality improvement (2018 - 2023)
Services for Congenital Heart Disease (CHD) span a patient’s lifetime, but their quality is currently mainly measured by 30-day survival following children’s heart surgery. 30-day survival, now almost 98%, is no longer a good proxy for quality. With the number of adults with CHD now outnumbering the paediatric population, the lack of routine monitoring of adult services and the quality of transition from child services is particularly problematic.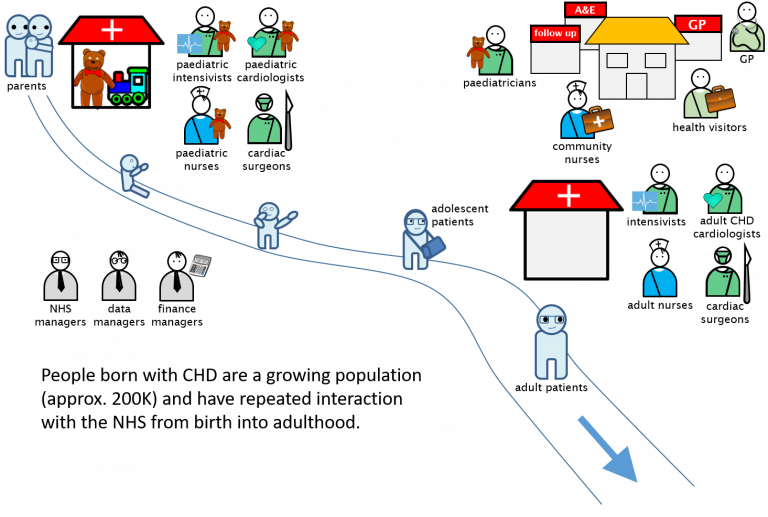
CORU's Christina Pagel and Sonya Crowe are leading a team of clinicians, commissioners, patient representatives and academics with both deep and wide experience of working to improve CHD services. With this project we hope to address the gaps in how quality is measured and reported in England for CHD.
Questions we will answer
By linking congenital heart disease, adult and paediatric intensive care audits, life status and HES (Hospital Episode Statistics) we will describe for the first time the trajectories of CHD patients through the NHS: what happens post operatively? How many hospital admissions are there? What happens as children become adults? Where does variation exist in the provision of care? What case mix adjustment is needed to evaluate the importance of that variation? What do we still not know and what do we know that is new? What are feasible (and desirable?) metrics of quality of care from the linked data set for quality improvement and quality assurance?
LAUNCHES QI is funded by the Health Foundation as part of their 2017 Insight programme.
- Long term outcomes (2013 - 2023)
- One of the most serious congenital heart defects that a baby can be born with is called “Hypoplastic Left Heart Syndrome” or HLHS for short. This means that the left side of the heart, which is meant to be the stronger side responsible for pumping blood around the body, does not work well. Left untreated it is always fatal. Babies born with HLHS need a series of operations in the first 5 or so years of life, starting in the very first weeks after birth.
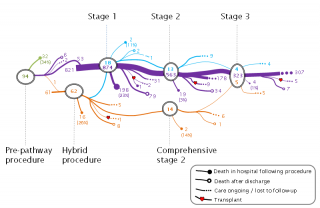
Previously, efforts have focused on improving early survival after the first, most dangerous, operation. Less has been known about what the longer term treatment pathway for HLHS patients looks like in practice. As survival has increased greatly for this condition over the last 20 years, understanding and communicating the longer term picture of what HLHS might mean to children has becoming increasingly important.
In a previous project led by Great Ormond Street Hospital, we used national audit data since 2000 to map out for the first time what happened to a national cohort of almost 1000 children born with HLHS. This showed the variety of possible pathways and longer term survival outcomes for children. We are now working on a new project with psychologists, parents and clinicians to convert this new knowledge into information for parents.
We are also expanding our work to other complex congenital heart conditions in a new three year project (2019-2022) funded by the British Heart Foundation and led by Dr Kate Brown at Great Ormond Street Hospital - we hope to shed new light on what treatment and outcomes look like for these children. Information about the use of data in this project can be found in the
Selected publications:
- Rogers, Pagel, Sullivan, Mustafa, Tsang, Utley, Bull, Franklin, Brown, “Interventions and outcomes in children with hypoplastic left heart syndrome born in England and Wales between 2000 and 2015 based on the National Congenital Heart Disease Audit”, Circulation, 136:1765-1767, 2017 (Open Access)
- Rogers, Pagel, Sullivan, Mustafa, Tsang, Utley, Bull, Franklin, Brown, “Interventional treatments & risk factors in patients born with hypoplastic left heart syndrome in England and Wales from 2000 to 2015”, Heart, doi: 10.1136/heartjnl-2017-312448, 2018 (Open Access)
GDPR notice:
For this study, UCL received information about children undergoing heart surgery in the UK since 2000 from the National Congenital Heart Disease Audit (NCHDA) managed by the National Institute for Cardiovascular Outcomes Research (NICOR). NCHDA did not provide any identifying information about you to UCL. We have used this information to understand more about longer term outcomes for children with Hypoplastic Left Heart Syndrome.
- Complications after children’s heart surgery (2014 - 2018)
Understanding the impact of important complications after children’s heart surgery (2014 - 2018)
Survival after children’s heart surgery is very high but it remains a risky surgery. Complications (e.g. infected wounds or injury to the organs) can have major short and long-term impacts on a child’s health and development. At the moment, it is not possible to estimate accurately the true scale of the impact of these complications on children and families, or of the considerable costs to the NHS.
This £1.1 million study funded by the National Institute of Health Research (NIHR) is taking place across 5 UK paediatric cardiac surgery centres and involves surgeons, representatives from the Children’s Heart Federation (the main umbrella patient and family group for children’s heart diseases), intensive care doctors, nurses, experts in clinical governance, data managers, psychologists, health economists, statisticians and other analysts.
After first spending a year selecting and defining which complications to measure, we have spent 2 years monitoring all children undergoing heart surgery at the five centres for complications. For some of these children, we are following them up 6 months after surgery to understand better the impact of complications, both in terms of clinical impact and impact on quality of life and costs (for families and the NHS).
CORU’s role includes: helping project management; leading data collection design and data capture; leading the selection phase of the project; developing ways to present the new information about morbidities and their impact to be useful to clinical teams, commissioners, audit bodies and families.
Selected publications:
- Pagel, Brown, McLeod, Jepps, Wray, Chigaru, McLean, Treasure, Tsang, Utley, “Selection by a panel of clinicians and family representatives of important early morbidities associated with paediatric cardiac surgery suitable for routine monitoring”, BMJ Open, 7:e014743. doi: 10.1136/bmjopen-2016-014743, 2017 (Open Access)
- Brown, Pagel, Brimmell, Bull, Davis, Franklin, Hoskote, Khan, Rodrigues, Thorne, Smith, Chigaru, Utley, Wray, Tsang, McLean, “Definition of important early morbidities related to paediatric cardiac surgery”, Cardiology in the Young, doi:10.1017/S1047951116001256 , 2016 (Open Access)
- PRAiS project (2010 - 2016)
Risk adjustment after children’s heart surgery and its communication: the PRAiS project (2010 - 2016)
Heart disease covers a wide range of disorders, from relatively minor to more severe conditions. Some hospitals take on more complicated conditions and tend to operate on children with a lower chance of survival. Also, every child is unique and may respond differently to treatment (surgery, drugs, postoperative care). We therefore would not expect all hospitals to have the same survival rate: we should take into account how ill the children were that the hospitals treated. In other words, if one hospital has a higher survival rate than another hospital this is not necessarily evidence that one hospital is better than the other – it could indicate that the second hospital treated children with more severe problems.
Since 2007, the national audit body (NCHDA) has published survival rates for certain types of procedure at each hospital. But the NCHDA did not publish overall survival rates for each hospital until 2013, because there was no clear way to put overall survival rates for each hospital into context. In 2010, CORU started work to develop a new statistical model to take into account the complexity of each case when considering a hospital’s survival rate. This led to the Partial Risk Adjustment in Surgery (PRAiS) formula, which has been used by the NCHDA since 2013 to publish hospital survival rates, taking account of case mix. CORU then worked with the audit body and three hospitals to build easy-to-use Excel software to allow hospitals to incorporate PRAiS risk adjustment in their local quality improvement processes. This software has since been purchased by all UK hospitals performing children’s heart surgery and is available for purchase from UCL Business.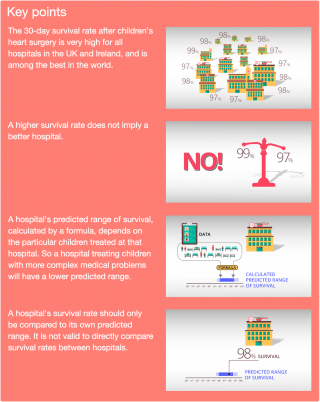
Over 15 months from 2015, CORU led an NIHR project where we updated the PRAiS model to incorporate more information about children’s diagnoses and other health conditions. In parallel, we led a second strand where we worked with Prof David Spiegelhalter in Cambridge, psychologist Dr Tim Rakow, the charities Sense about Science and the Children’s Heart Federation to co-develop a new website to explain how PRAiS is used by national audit. Most importantly, we worked with parents to explain what PRAiS can and can't be used for. The website, http://childrensheartsurgery.info, was launched in June 2016 to great acclaim.
Selected publications:
- Brown, Rogers, Barron, Tsang, Anderson, Tibby, Witter, Stickley, Utley, Crowe, English, Franklin, Pagel, “Incorporating Comorbidity Within Risk Adjustment for UK Pediatric Cardiac Surgery”, Annals of Thoracic Surgery, doi: 10.1016/j.athoracsur.2016.12.013, 2017 (Open Access)
- Rogers, Brown, Franklin, Ambler, Anderson, Barron, Crowe, English, Stickley, Tibby, Tsang, Utley, Witter, Pagel, “Improving Risk Adjustment for Mortality After Pediatric Cardiac Surgery: The UK PRAiS2 Model”, Annals of Thoracic Surgery, doi: 10.1016/j.athoracsur.2016.12.014, 2017 (Open Access)
- Pagel, Jesper, Thomas, Blackshaw, Rakow, Pearson, Spiegelhalter, “Understanding Children’s Heart Surgery Data: A Cross-Disciplinary Approach to Codevelop a Website”, Annals of Thoracic Surgery, doi:10.1016/j.athoracsur.2016.11.080, 2017 (Open Access)
- Pagel, Crowe, Brown, Utley, “The benefits and risks of risk-adjustment in paediatric cardiac surgery”, Heart, 100:528-529, doi:10.1136/heartjnl-2013-304848, 2013. (Open Access)
- Pagel, Utley, Crowe, Witter, Anderson, Samson, McLean, Banks, Tsang, Brown, “Real time monitoring of risk-adjusted paediatric cardiac surgery outcomes using Variable Life Adjusted Display: implementation in three UK centres”, Heart, doi:10.1136/heartjnl-2013-303671, 2013
- Crowe, Brown, Pagel, Muthialu, Cunningham, Gibbs, Bull, Franklin, Utley, Tsang, “Development of a diagnosis and procedure based risk model for 30-day outcome following paediatric cardiac surgery”, Journal of Thoracic and Cardiovascular Surgery, 145:1270-8, 2013
GDPR notice:
For this study, UCL received information about children undergoing heart surgery in the UK since 2000 from the National Congenital Heart Disease Audit (NCHDA) managed by the National Institute for Cardiovascular Outcomes Research (NICOR). NCHDA did not provide any identifying information about you to UCL. We have used this information to improve the statistical formula used by NCHDA to report survival outcomes for UK hospitals performing heart surgery on children.
- Infant deaths following cardiac surgery (2012 - 2016)
Infant deaths following cardiac surgery (2012 - 2016)
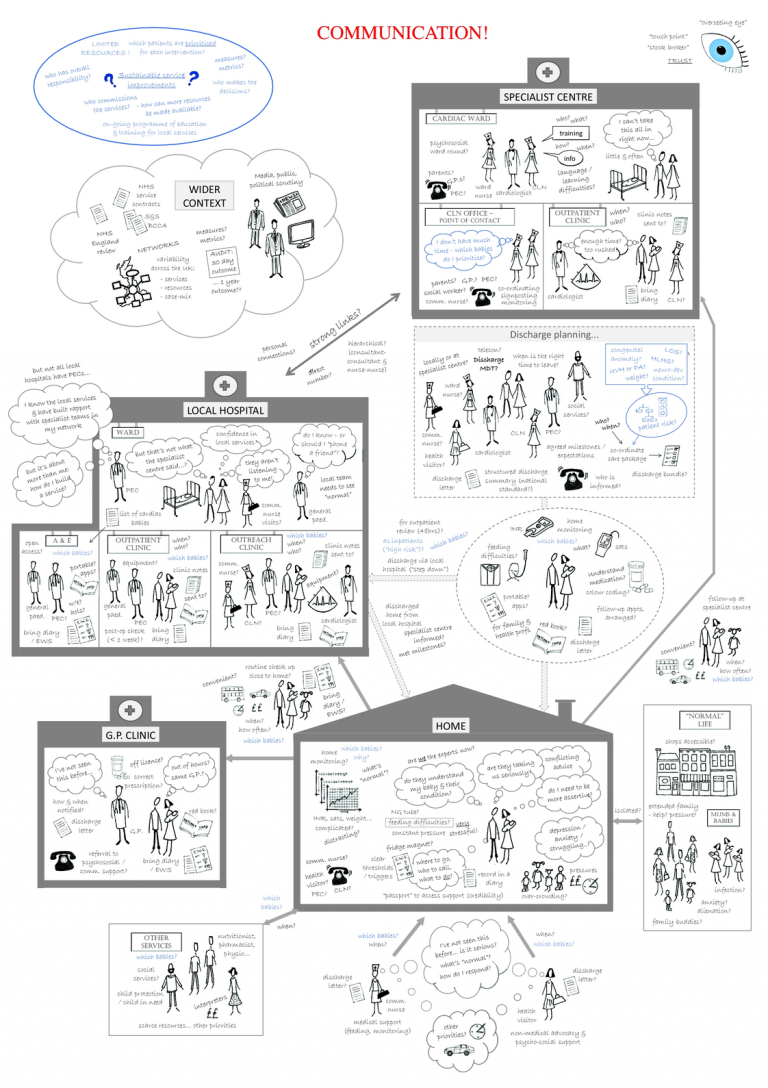
The number and complexity of infants with congenital heart disease surviving intervention and requiring care in the community following discharge from the specialist surgical centres are increasing, yet research focuses predominantly only on their in-hospital and short-term outcomes. CORU was part of an NIHR multidisciplinary programme of research conducted in response to concerns over out-of-hospital mortality levels in this patient population, variability in the provision of services at and following discharge, and barriers to care experienced by some families.
We worked closely with clinicians at Great Ormond Street Hospital and elsewhere to link and analyse two national audit datasets (NCHDA and PICANet), showing for the first time that adverse outcomes in the year after discharge are of similar magnitude to in-hospital mortality in the UK. We also used the linked data to identify subgroups of infants with congenital heart disease that might benefit from different interventions following discharge after surgery because of their differing profiles of risk. Using soft systems analysis, CORU then helped an expert group to utilise this analysis in prioritising and targeting recommendations for improving services for these patients. The overall project (The Infant Heart Study) was led by Dr Kate Brown at Great Ormond Street Hospital.
The research has attracted considerable attention from healthcare commissioners, providers and charities involved in services for infants undergoing cardiac surgery and have influenced national decision-making about service standards and commissioning in this area. Our paper describing the innovative application of combined ‘soft’ and ‘hard’ operational research methods in this project was featured on the front cover of BMJ Quality and Safety and has attracted international attention among both academic and practitioner audiences.
Selected publications:
- Crowe, S., Brown, K., Tregay, J., Wray, J., Ridout, D., Knowles, R.L., ...Utley, M. (2017). Combining qualitative and quantitative operational research methods to inform quality improvement in pathways that span multiple settings. BMJ Quality and Safety, 26(8). doi:10.1136/bmjqs-2016-005636 (Open Access)
- Crowe S, Ridout D, Knowles R, Tregay J et al. (2016), Death and emergency readmission of infants discharged after interventions for congenital heart disease: A national study of 7643 infants to inform service improvement. Journal of the American Heart Association, 5(5). https://doi.org/10.1161/JAHA.116.003369 (Open Access)
- Crowe S, Knowles R, Wray J, Tregay J et al. (2016), Identifying improvements to complex pathways: evidence synthesis and stakeholder engagement in infant congenital heart disease. BMJ Open, 6(6), e010363 (Open Access)
- Knowles, R.L., Ridout, D., Crowe, S., Bull, C., Wray, J., Tregay, J., ...Parslow, R.C. (2016). Ethnic and socioeconomic variation in incidence of congenital heart defects. Arch Dis Child, doi:10.1136/archdischild-2016-311143 (Open Access)
- Brown K, Wray J, Knowles R, Crowe S et al. (2016), Infant deaths in the UK community following successful cardiac surgery: building the evidence base for optimal surveillance, a mixed-methods study. Health Services and Delivery Research, 4(19) (Open Access)
 Close
Close

