Mayor Lab

Journal covers from the Mayor Lab

Swarming: A model for collective cell migration (Carmona-Fontaine et al. 2011 Dev Cell 21, 1026 - 1037)

Short-range chemotaxis drives epiboly (Szabó et al. 2016 Dev Cell 37, 213 - 225)

Colour-coded temporal projection of migratory Neutral Crest cells. Photo by C. Carmona-Fontaine

Collective chemotaxis of Neural Crest cells. Cell body in yellow protrusions in red (Theveneau et al. 2010 Dev Cell 20, 39-53)

Migration of Neural Crest zebrafish in vivo. Colour-coded temporal projection. Photo by R. Mayor

Neural Crest cells form swarms resembling bird flocks or schools of fish. After M.C. Escher's. (Carmona-Fontaine et al 2011, Dev Cell 21, 1026 - 1037)

Colour-coded temporal projection of migratory Neural Crest cells. Photo by C. Carmona-Fontaine

Collective chemotaxis of Neural Crest cells. Cell body in green and protrusions in magenta (Theveneau et al. 2010, Dev Cell 20, 39 - 53)

Microtubules in migrating Neural Crest. Photo by R. Moore
What is the Neural Crest?
The Neural Crest is a group of cells found in all vertebrate embryos for a transient period of time during their development. It forms in the neural folds at the border of the neural plate.
While the neural plate eventually becomes the Central Nervous System, the Neural Crest gives rise to the Peripheral Nervous System, as well as the cartilage, bone and muscle in the face and neck, pigmented cells in the skin, several endocrine glands and part of the heart. It is this extraordinary ability of the Neural Crest to become many different types of cell that has attracted the attention of many biologists.
The second astonishing characteristic of neural crest cells is that they are able to migrate very long distances in the embryo. The neural crest has been called the "explorer of the embryo" as it is one of the embryonic cell types that migrates most during development, eventually colonizing almost every tissue.
Why Study the Neural Crest?
Although the neural crest is a discrete and transient structure that comprises only a few cells, it embodies many of the crucial issues of Developmental Biology. Questions related to embryonic induction, morphogenesis, differentiation, and even evolution have to be addressed in order to develop an understanding of neural crest development.
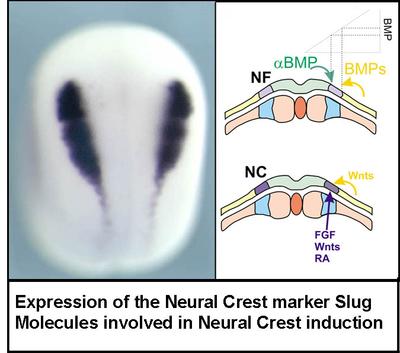
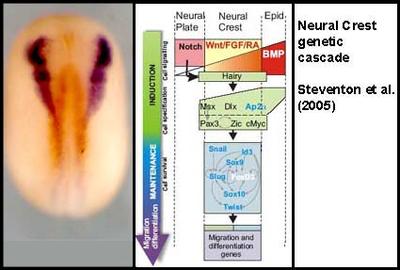
Induction
The neural crest is formed at the border of the neural plate.
- How is this group of cells induced at a particular position?
Morphogenesis
During and after the closure of the neural tube, the neural crest cells start one of the most dramatic morphogenetic events during early embryogenesis: they are segregated from the neuroepithelia, and they migrate throughout the embryo.
- How is this segregation and cell migration controlled?
- Is it similar to what happens during tumor progression and metastasis?
Differentiation
The neural crest differentiates into a prodigious array of cell types.
- How is this differentiation controlled?
- What determines the fate of each of the neural crest derivatives?
Evolution
Finally, the neural crest represents a key tissue in vertebrate evolution, because many of the typical features of vertebrates (e.g., the jaw) are neural crest derivatives.
- How did this small group of cells emerge during evolution?
- How was it able to play such an important role in vertebrate evolution?
These are questions that have fascinated developmental and evolutionary biologists for more than a century.
Human pathologies related to Neural Crest
Several human pathologies are produced by a failure of neural crest development. The defect can occur during the early specification of the neural crest as part of its migration or differentiation.
DiGeorge syndrome
One of the abnormalities associated with the cephalic neural crest is DiGeorge syndrome. Patients with this syndrome have an abnormal migration of the neural crest in the pharyngeal arches. They have hypocalcemia that arises from defects in the parathyroid glands, thymic hypoplasia, and outflow tract defects of the heart. The facial anomalies include low-set ears, a small mouth and philtrum (the space between the upper lip and the nose), and cleft palates.
Hirschprung disease
A failure of vagal neural crest cell migration to the colon results in the absence of enteric ganglia and, thus, in the absence of peristaltic movement in the bowel. The human pathology associated with this failure is called Hirschsprung disease (or megacolon).
Animal models to understand disease
Several animal models have been developed that reproduce some or all aspects of these and other neural crest pathologies. The most frequent organism used to study these pathologies is mouse, as it is a mammal.
However, organisms such as Xenopus and zebrafish have also been very useful for the study of the role of specific genes on a particular phenotype that mimics the human syndrome. This is a very active area of research that is growing very quickly.
Understanding the normal cellular/molecular/genetics mechanisms of neural crest development in different animal models will be essential for the further understanding of these human syndromes.
Focus: Development of the Neural Crest
The primary aim of our research group is to elucidate the mechanism that underlies the development of the Neural Crest. We would like to know how Neural Crest cells acquire their identity within the ectoderm and how their migration and differentiation is controlled.
Model system: zebrafish and frog
We study neural crest development in zebrafish and frog ( Xenopus laevis) embryos as these two animal models offer several complementary advantages. Zebrafish embryos are transparent and the migration of the Neural Crest is easy to visualize. In addition it is a system suitable for genetic studies. Xenopus embryos are bigger and easier to manipulate.
Our lab belongs to the UCL Research Department of Cell and Developmental Biology and the UCL Neuroscience Domain.
 Close
Close