UCL support towards commercial adoption and uptake of biologics therapeutics into the NHS.
 GOING TO MARKET:
GOING TO MARKET:
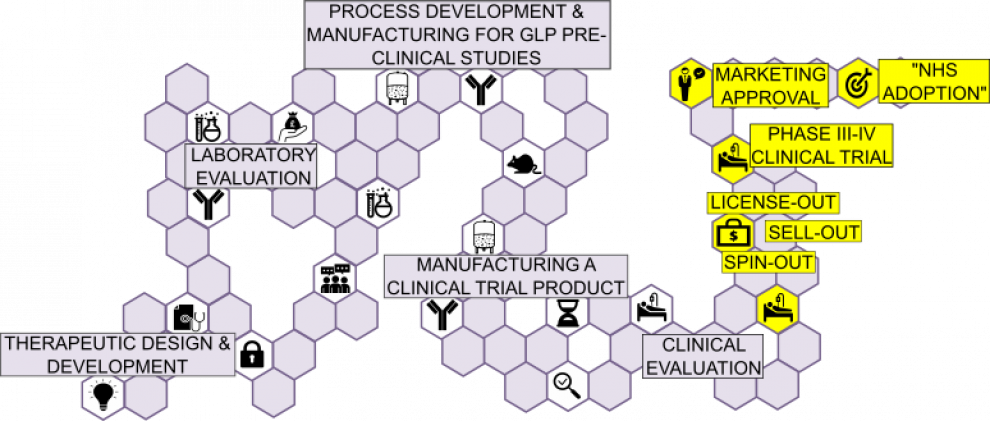
Having completed the primary clinical safety and efficacy evaluation, regulatory authorities require manufacturers to:
- Undertake further Phase III and IV clinical trials in the wider population
- Establish potency assay
- Lock-down commercial manufacturing process
- Validate all in-process and release assays
- Regulatory body (MHRA/EMA/FDA) approval
- NICE approval.
In practice, the resources required for such an undertaking are often beyond UCL and the therapeutics would be expected to be commercially adopted in one of three ways:
- SELL-OUT: Biologic sold in entirety for one-off deal to a company/device manufacturer
- LICENCED-OUT: UCL retains the biologic but grants license to company for marketing
- SPIN-OUT: UCLB investment establishes a separate commercial entity to market the biologic
 NHS ADOPTION:
NHS ADOPTION:
Key tips to facilitate adoption - Continuously undertake internal and external stakeholder review of the biologic:
- The Steering Committee should meet regularly (at least every 6 months)
- Ensure the identification and measurement of tangible data that will support adoption (e.g. outcomes meaningful to ward managers, hospital laboratory managers)
Be mindful of the key ‘facets’ developed for successfully adopted biologics:
- FISCAL OPPORTUNITY: Clearly defined & attractive market potential
- SYSTEM OPERATIONS: Awareness & solutions to operational challenges of delivering the healthcare
- COMMUNICATION: Effective publicity of the biologic throughout its lifetime
- HUMAN FACTORS: Minimized use-related hazards, risks & inconvenience wherever practically possible
- PROOF: Provision of ‘work as done’ evidence (i.e. in both clinical & laboratory scenarios)
- IPR: Secure intellectual property rights
UCL Support:
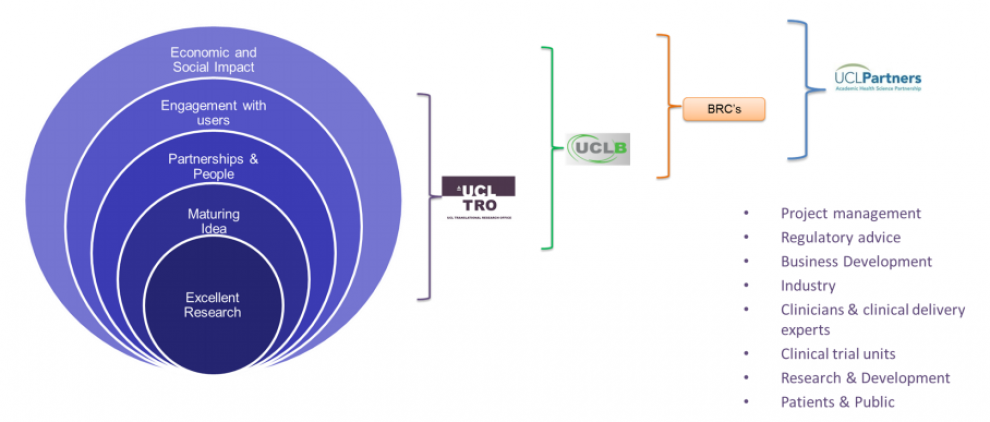
UCL’s Translational Research Office (TRO) builds on an increasingly vibrant translational culture across the wider university community by providing integrated support for translational research and industrial partnerships.
UCL has implemented and is growing strategic platform partnerships to facilitate the accelerated development, commercialisation and adoption of therapeutics into the NHS.
 Close
Close

