UCL support towards commercial adoption and uptake of medical devices into the NHS.
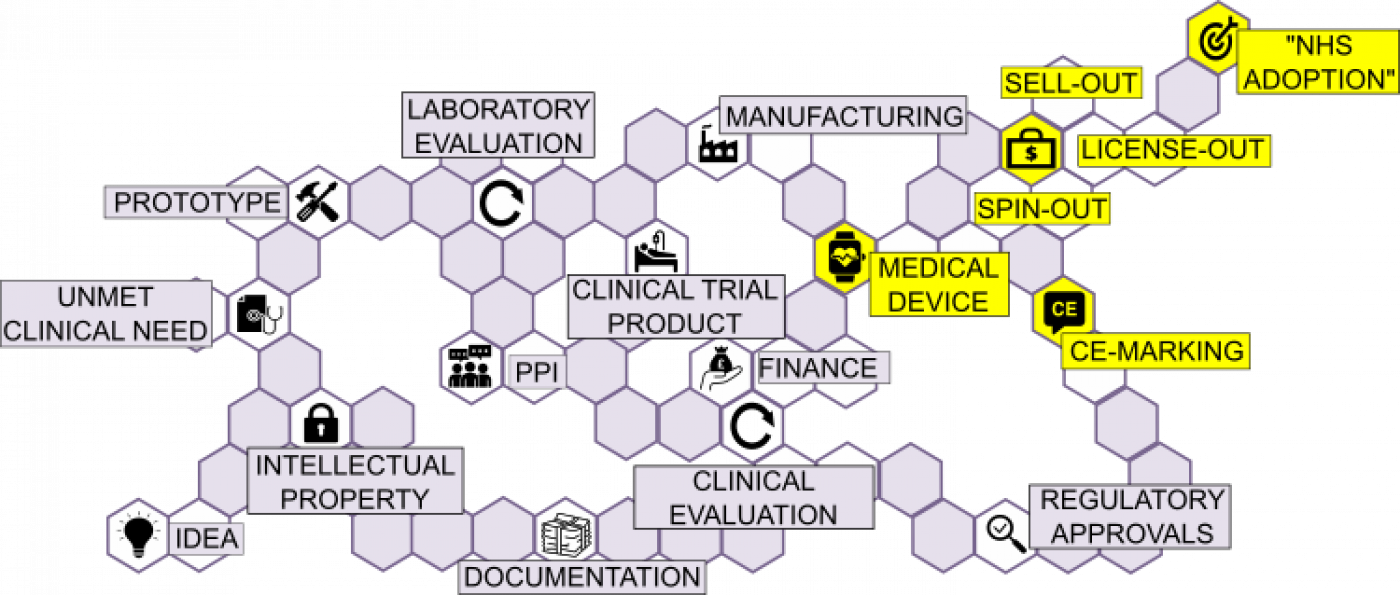
 GOING TO MARKET:
GOING TO MARKET:
Having completed the primary clinical and performance evaluation, release of a new Medical device onto the market then requires manufacturers to:
- Undertake further, post market surveillance of safety in the wider population.
- Establish Vendor chains for after-sales training and service provision, problem reporting, tracking distribution and implementing recall procedures if required.
In practice, the resources required for such an undertaking are often beyond UCL and the Device would be expected to be commercially adopted:
- SELL OUT: Device sold in entirety for one-off deal to a company/manufacturer
- LICENSED OUT: UCL retains the device but grants license to company for marketing
- SPIN-OUT: UCLB investment establishes a separate commercial entity to market the device
 CE-MARKING:
CE-MARKING:
“Having a patent on a device is effectively useless without a CE Mark”
CE-marking is a declaration of conformity with the EU’s Medical Devices Directive (MDD) and is:
- NOT a requirement for clinical trial/evaluation (NB. such evidence may be required for CE-marking)
- A requirement to place a device on the market in the EU
Devices are grouped by increasing risk, with increasing assessment requirements:
| Risk Class | Risk Description | Examples |
|---|---|---|
| Class I basic (non-measuring, non sterile) | Low | Reusable surgical instruments (e.g. forceps, scissors, scalpel to be sterilised locally); ECG electrodes; Beds; Wheelchairs |
| Class I measuring | Low | Stethoscope; Thermometer; Non-invasive blood pressure; Volumetric urine bag |
| Class I sterile | Low | Sterile dressing (non-medicated); Examination gloves (e.g. item is supplied sterile then discarded) |
| Class IIa | Medium-Low | ECG machine (recording only); diagnostic ultrasonic equipment; Hypodermic needles (NB. invasive, but exception as used only transiently); Suction equipment; Fridge (blood) |
| Class IIb | Medium-High | ECG machine (continuous - with alarms [e.g. ICU]); Infusion pumps; Invasive blood pressure, Ventilators; orthopaedic implants; Contraceptives; Haemodialysis machine; X-ray machines |
| Class III | High | Drug eluting cardiac stents; Heart valves; Ultrasound equipment for use in interventional cardiac procedures |
| Active Implantable Medical Device (AIMD) | High | Implantable pacemaker; Implantable defibrillator |
Class I devices can be self-certified (i.e. manufacturer ensures compliance with all the relevant essential requirements of the Medical Device Directive (MDD) and provides written statement to this effect), the device can be CE marked and then placed on the market. Although not required to ISO13485 standard, an appropriate Quality Management System is required to
develop and maintain a Class I device. CE mark holding demands continual effort (e.g. post-launch surveillance) and UCL usually defaults to an option with an external company holding the CE mark.
 NHS ADOPTION:
NHS ADOPTION:
Continuously undertake internal and external stakeholder review of the Device:
- The Device Development Group should meet regularly (at least every 6 months)
- Ensure the identification and measurement of tangible data that will support adoption (e.g. outcomes meaningful to ward managers, hospital laboratory managers)
Be mindful of the key ‘facets’ developed for successfully adopted devices:
- FISCAL OPPORTUNITY: Clearly defined and attractive market potential
- SYSTEM OPERATIONS: Awareness and solutions to operational challenges of delivering the healthcare
- COMMUNICATION: Effective publicity of the device throughout its lifetime
- HUMAN FACTORS: Minimized use-related hazards, risks and inconvenience wherever practically possible
- PROOF: Provision of ‘work as done’ evidence (i.e. in both clinical and laboratory scenarios)
- IPR: Secure intellectual property rights (IPR) as early as possible, then drive for market adoption as soon as possible (do not delay by developing next device generation)
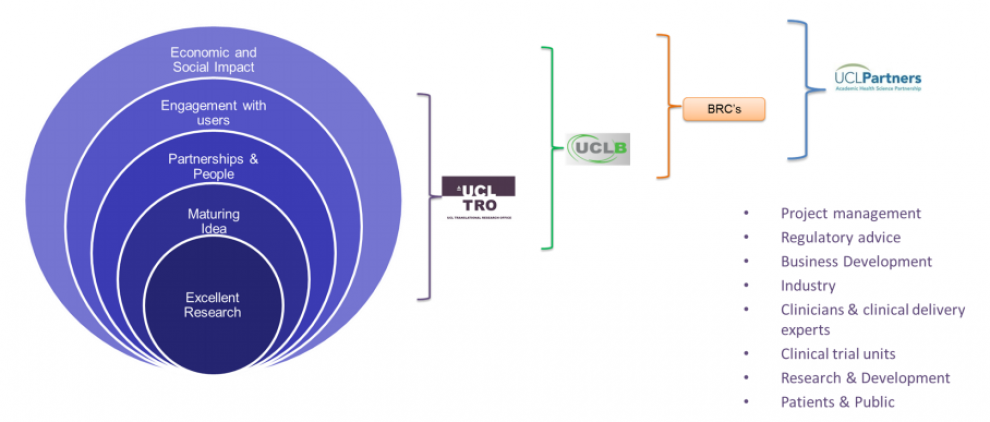
UCL Support:
UCL’s Translational Research Office (TRO) builds on an increasingly vibrant translational culture across the wider university community by providing integrated support for translational research and industrial partnerships.
UCL has implemented and is growing strategic platform partnerships to facilitate the accelerated development, commercialisation and adoption of its medical devices into the NHS.
 Close
Close

