Lessons learnt: The Mechanics Behind the Rapid Translation of the CPAP Device
This article provides an overview of the UCL Ventura CPAP story as told in the first COVID TINs Seminar Series, highlighting mutual respect & collaboration as the key factor for biomedical translation
14 July 2020
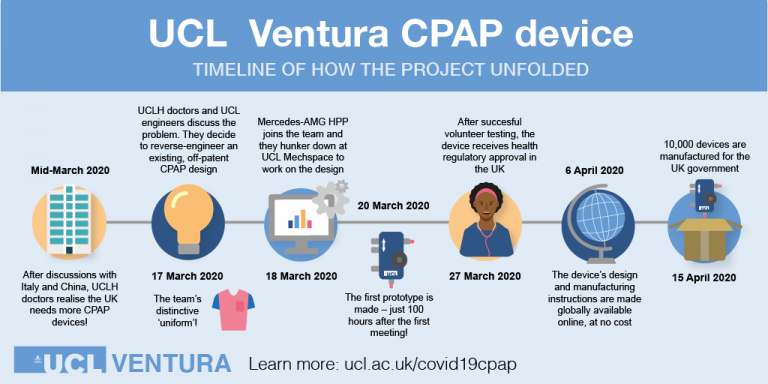
On Wednesday 1st July 2020, we kicked off the COVID-19 TINs Seminar Series, hearing from the UCL Ventura team and their remarkable story developing the continuous positive airway pressure (CPAP) device. The seminar included the perspectives of several partners involved in the project (directly & indirectly), documenting how they worked collaboratively to navigate the translational pathway with great efficiency, to allow the product to reach hospitals so rapidly in a time of such need.
Before handing over to the panellists, Professor David Lomas (Vice Provost (Health), UCL School of Life and Medical Sciences), who chaired the session and played a prominent role in the project progression, highlighted that the heart of this success story was a partnership that goes back many years, between UCLH, UCL and Mercedes.
Putting the clinical story in context, Professor Mervyn Singer (Professor of Intensive Care Medicine, UCL/UCLH) introduced the idea that in order for UCLH to be prepared as a hospital to support the expected influx of patients, there was a need to keep people off ventilators and out of intensive care. This was not in line with the national advice and government call to come up with new ventilators, but the learnings from China and Italy’s experiences suggested efforts should be focussed on keeping people off ventilators by using existing CPAP devices. Mervyn therefore engaged the engineers at UCL to modernising the technology by reverse engineering the devices and making them in bulk.
Professor Lomas then introduced Pia Larsen (Director Procurement & Supply Chain, UCLH) whose responsibilities included supporting the set-up of the high dependency respiratory unit at UCLH. This included gearing up the new ward to have sufficient consumables to support patients when on the CPAP devices – something which became increasingly difficult as required components came in short supply. As the procurement team suggested alternatives to get around this (humidified breathing circuits instead of dry, for example), Pia emphasised the reliance on quick feedback from clinicians such as Dr Singer, to identify the most appropriate consumables. It was this trustworthy, respectful relationship between the procurement team and physicians that was the key success factor for the respiratory unit working as efficiently as it did. Getting feedback from clinicians in such a prompt and agile way allowed them to make decisions and source equipment quickly.
Passing onto Professor Becky Shipley (Director, UCL Institute of Healthcare Engineering) and Dr Tim Baker (UCL Mechanical Engineering), the engineering team involved in the project, they highlighted the challenges involved in producing a device that wasn’t exactly what the government had asked for at the time. The idea to focus on re-engineering the old CPAP device was attractive to the engineering team. Agreeing that they thought it to be achievable, Professor Shipley moved to involvethe MHRA straight away to help the device move quickly through regulatory approvals. From the manufacturing view point with the realisation of the volume of devices that would be required, Dr Baker immediately thought of his contacts within the motorsport industry, which is where Mercedes got involved.
The next part of the webinar followed the journey of the CPAP device where it really comes to life, with the engineering team working around the clock with Mercedes (represented by Andy Cowell, Managing Director, Mercedes AMG High Performance Powertrains Ltd) to produce 10,000 kits ready to dispatch to the NHS within 4 weeks. This part of the story is extremely intricate, not to mention exciting, and is best told by the engineering and manufacturing teams themselves - view the webinar recording here.
Something that hit home from Andy’s comments, was the shared passion from the entire team working towards the same common goal which was driven by patient impact/benefit rather than financial gain. There was no need for introductions, NDA’s or lengthy commercial terms or financial discussions as there would usually be, it was just a case of “roll your sleeves up and get stuck in”.
The webinar then moved onto some entirely different but equally important partners needed for the progression of any devices project, starting with regulator Dr Neil McGuire (Senior Clinician Devices, MHRA). The regulatory process is in place to protect patients and often this approval process can take a long time – something which they didn’t have in this situation. Dr McGuire was respectfully honest in admitting that as a regulator, projects are often approached with great suspicion where there are usually a mix of motivations associated with a products development. However in this case, trust was developed early and Dr McGuire could see that in this unique partnership driven to re-engineer an already approved safe device that despite the incredible pace everything was being performed to the highest standards and was impressed with the incredible motivational spirit of the entire team and sincerity to benefit patients.
Marina Santilli (Associate Director (Physical Sciences & Engineering), UCLB) represented a Tech Transfer Office perspective, and discussed the complications associated with creating a free of charge, open source licence for Intellectual Property developed jointly between UCL and Mercedes, and how the team worked to achieve this. The storefront (e-lucid platform) developed supports the volume of requests received for the open access UCL Ventura CPAP designs, and UCLB are also offering to host additional COVID technologies from UCL or other universities through this storefront.
To wrap up this tremendous journey, Dr Julius Mugwagwa (Lecturer in Innovation and Development, UCL STEaPP) highlighted the report he produced to evaluate and understand the critical points that allowed this project to move so rapidly. Dr Mugwagwa then introduced an audience poll to see what the audience opinions were on the “the single most important factor in the project's success?” Poll results below:
- 33% - Establishing mutual trust and respect between all partners
- 22% - Having an already established network of partners to mobilise rapidly
- 22% - Responding to a global crisis/priority gave a clear sense of urgency
- 17% - Willingness to accept risks on behalf of the partners (e.g. funding, indemnity, reputation)
- 6% - Hospital usability and timely mass manufacture prioritised early in device design
This was followed by a panel discussion with questions submitted by the audience, available to view in the webinar recording accessible here.
Hearing the story from multiple perspectives was inspiring yet humbling to listen to. It is clear that collaboration and a “can-do” attitude was key to the success of this project, with all partners trusting each other and in constant communication, fulfilling required tasks in order to work together and support the project. It is this joint effort throughout a network of connections that propelled the project to market so rapidly.
This was reiterated in the closing slide of the session, where Professor Rebecca Shipley, Devices & Diagnostics TIN Chair, highlighted joining a UCL Therapeutic Innovation Network as an opportunity to build connections in the area of your modality of interest, learn best practice from colleagues who have experience of the translational pathway and access a platform for multidisciplinary and collaborative research to bridge the gap between academia, industry and partner hospitals.
How to get involved – join a TIN:
- Academia – Contact the relevant TIN Coordinator to explore opportunities (Devices: Dr Laurent Dupays l.dupays@ucl.ac.uk)
- Industry – book a TIN Design Consultation to prioritise interest areas, identify ideal participants and develop interaction objectives with our specialist team.
View the full webinar recording
 Close
Close

