Working with Wild-type (Unmodified) Agents
This page provides guidance on working with Wild-type biological agents at UCL.
Before starting work
Wild type (unmodified) microorganism risk assessment is carried out to identify the necessary controls that are required to restrict the exposure of employees to hazard group 2 and hazard group 3 microorganisms or materials such are tissues derived from humans or animals that could harbour pathogens hazardous to human health or the environment.
The wild type microoorganism risk assessment must consider the following:
- Identification of the Hazard Group (HG) of the microorganisms.
- Information on Hazard Groups can be found in the Approved List of biological agents.
- The Containment Level (CL) required for handling the microorganisms.
- Additional security requirements are required if the microorganism is listed in Schedule 5. [does this relate to purchasing, storing etc?)
- Safe storage of the biological agent.
- Is the microorganism listed under the Specified Animal Pathogen Order. [what are the implications?].
Speak to your local Genetic Modification Safety Officer (GMSO) or the Biological and Chemical Safety team to ensure that you are aware of the requirements before ordering any biological agents including wildtype (unmodified) microorganisms as well as tissues, fluids and other materials derived from humans or animals
Mandatory training
Before working with Hazard Group microorganisms, you must complete the formal training:
> Principles of laboratory safety
> Principles and practice of biosafety
Areas working with HG2 and HG3 microorganisms will have specific training for their laboratories and activities.
Guidance on completing risk assessment
riskNET: Risk Assessment of Biological agents and plants provides guidance on completing and approving biological agent and plant risk assessments, covering genetically modified and wild type work activities.
- Work with Hazard Group 1
Hazard Group 1 work involves biological agents that have not been genetically modified and are unlikely to cause diseases in a healthy individual. Activities with Hazard Group 1 wild-type biological agents do not require enhanced biosafety controls and can be used in most labs as long as good lab safety principles are followed.
For advice on completing the form refer to the Risk assessment page. Once the assessor has completed, the risk assessment should be approved by the department. The risk assessment is typically assigned to the PI in control of the work and the local Genetic Modification Safety Officer (GMSO). If the PI is the assessor, then a peer with knowledge and experience of this type of work can review and approve the risk assessment to confirm the technical detail and the controls to be implemented as suitable.
Copying an existing risk assessment
MediaCentral Widget Placeholderhttps://mediacentral.ucl.ac.uk/Player/a3GEH57g Creating a new risk assessment
MediaCentral Widget Placeholderhttps://mediacentral.ucl.ac.uk/Player/GB1He3JJ 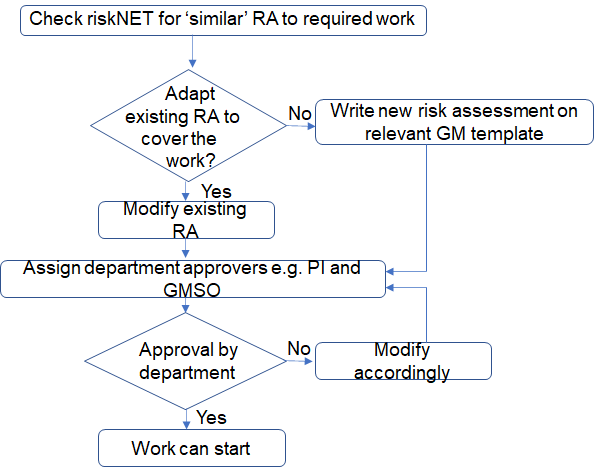
- Work with Hazard group 2 and 3
Before starting work for the first time and bringing the biological agent on-site:
- Identify the Hazard Group and Containment Level for the microorganisms you will be using to ensure the right level of containment is available. Information on the hazard groups can be found in the Approved List of biological agents (gov.uk).
- If the biological agent is listed in Schedule 5 and/or Specified Animal Pathogen Order there are additional security requirements. Speak to your Departmental Safety Officer and the Biological and Chemical Safety team to ensure that you are aware of the requirements before ordering.
- Check riskNET to see if there is a risk assessment in place which is similar to the work proposed. If a similar risk assessment exists, copy the risk assessment to get a new reference number and amend the risk assessment to reflect the new work.
> How to create a new risk assessment from an existing risk assessment
MediaCentral Widget Placeholderhttps://mediacentral.ucl.ac.uk/Player/a3GEH57g - If a risk assessment doesn't exist in riskNET, select the relevant specialist risk assessment form for Hazard Group 2 or 3. For advice on completing the form refer to the Risk assessment page.
> How to create a new risk assessment
MediaCentral Widget Placeholderhttps://mediacentral.ucl.ac.uk/Player/GB1He3JJ - When completing the risk assessment the assessor identifies the approvers who will authorise the assessment. Typically this is the PI who is in control of the work and the local Genetic Modification Safety Officer (GMSO). If the PI is the assessor, then a peer with knowledge and experience of this type of work can approve the risk assessment. This will ensure the technical detail and the controls to be implemented are suitable.
- Once the risk assessment has been authorised by the approvers listed in the risk assessment, HG 2 and HG 3 risk assessment will be reviewed by the GMBSSC. Only when this approval is provided can the work commence.
Additonal approval for HG 3 and named HG2 microoorganisms
Once the approvers listed in the GM risk assessment have approved the risk assessment, approval is also required by the GMBSCC before the risk assessment is fully authorised.
Work with the following agents requires approval from the Health and Safety Executive before the GMBSCC can authorise the risk assessment. This is because these HG2 microorganisms are spread by aerosol. Submission to the HSE requires the completion of a CBA1 form which the Biological Safety Officer will issue to you. The HSE requires 20 days to review the risk assessment. If no concerns are raised the risk assessment will be fully authorised and work can commence.
Bordetella pertusis- Corynebacterium diptheriae
Work with Hazard Group 2 and 3 microorganisms cannot begin until a risk assessment has been authorised by the GMBSCC. Only once Risk Assessment has been fully authorised can work commence.
- Neisseria menigititis
-
HG 2 risk assessment process flow
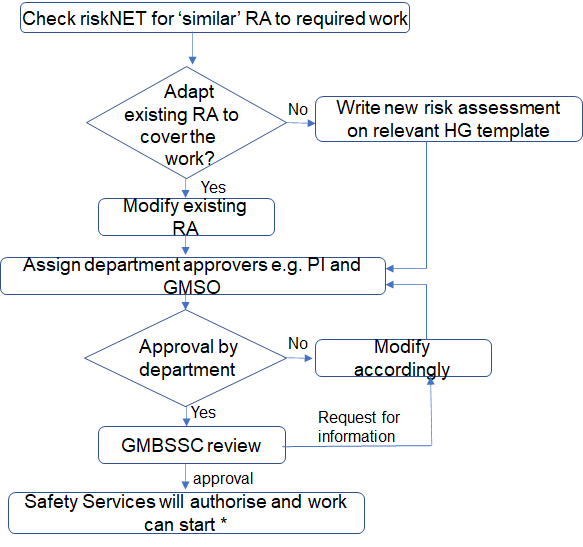
HG 3 risk assessment process flow
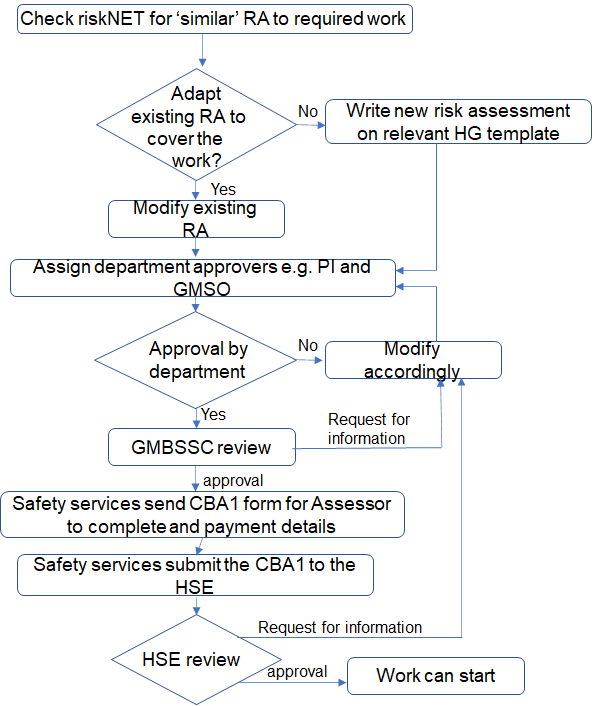

Last updated: Tuesday, January 24, 2023
 Close
Close


