Transporting Infectious and Biological Material
Transport of biological material such as diagnostic samples, pathogens or infectious substances is highly regulated. This page provides information and how to access advice and training.
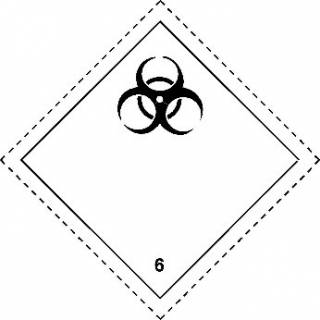
- Non-pathogenic, wild-type micro-organisms.
- Waste or biological material that has been inactivated so it no longer poses a risk of infection.
- Substances transported in a form whereby any pathogens present have been neutralised or inactivated such that they no longer pose a health risk (e.g. material fixed in formaldehyde).
- Dried blood on absorbent material.
- Environmental samples (including food and water samples) not considered to pose a significant risk of infection.
- Naked nucleic acid, plasmids or liposome gene delivery systems.
If the material does not fall into the exempt categories above and is Genetically Modified and/or an Infectious substance, transport regulations apply.
In order to correctly classify the substance you wish to transport, please email the UCL Dangerous Goods Safety Advisor.
Transporting guidance
The guidance below will help you prepare while you wait for detailed advice.
- Category A infectious substances
This category includes higher-risk agents and has the definition: "An infectious substance, which is transported in a form that, when exposure to it occurs, is capable of causing permanent disability, life-threatening or fatal disease in otherwise healthy humans or animals"
An indicative list is contained in the transport regulations. Please refer to this list to verify if the substance you wish to transport is Category A or not. New or emerging pathogens which do not appear on the indicative list but which meet the definition above must be transported as Category A. Avian Influenza is one such example. These substances are given. the classification 'UN2814 Infectious Substance, Affecting Humans'.
> The indicative list (IATA website)
- Category B infectious substances
Includes any Infectious substances that do not meet the criteria of category A. In addition to pathogens, this category includes human or animal material such as excreta, secreta, blood, tissue, tissue fluids and body parts transported for research, diagnosis, disease treatment or prevention. These substances are given the classification 'UN 3373 Biological Substance Category B'.
- Genetically modified organisms
Genetically Modified Organisms (GMOs) and Genetically Modified Micro-organisms (GMMs) that meet the definition of an infectious substance shall be classified as either Category A or Category B (as defined above).
GMMs that do not meet the definition of an infectious substance but are capable of altering animals, plants or microbes in a way not normally the result of natural reproduction are classified in Class 9 - 'Miscellaneous Dangerous Goods' and consigned as 'UN 3245 Genetically Modified Micro-organisms'.
Packaging
- Category A infectious substances
You must consult the UCL Dangerous Goods Safety Advisor for advice before packaging and transporting Category A Infectious Substances as specific legislative requirements and security controls apply.
- Category B infectious substances
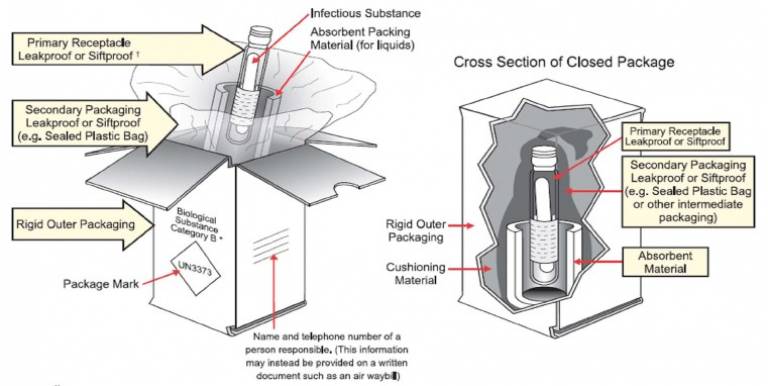
In packaging Category B substances, you must include the following:
- Leak-proof primary receptacle(s).
- Leak-proof secondary container.
- Absorbent material between primary receptacle(s) and secondary container (not required for solid samples). Must be sufficient to absorb contents of all primary receptacles.
- Primary receptacles must be individually wrapped or separated to prevent contact between them.
- Rigid outer packaging of adequate strength for its capacity, weight and intended use Itemised list of contents to be enclosed between secondary container and outer packaging.
An illustration of the packaging requirements is shown below.
Category B Biological Substances (which do not require dry ice) may be sent through Royal Mail/The Post Office within the UK.
- Dry Ice
When used, ice or dry ice (solid carbon dioxide) shall be placed outside the secondary packaging / inside the outer packaging or in an overpack. Interior supports must be provided to ensure that the secondary packaging remains in the original position after the ice or dry ice (solid carbon dioxide) has dissipated.
If ice is used, the outside packaging must be leak-proof. If dry ice (solid carbon dioxide) is used, the packaging must be designed and constructed to permit the release of carbon dioxide gas to prevent a build-up of pressure that could rupture the packaging.
The primary receptacle and secondary packaging must maintain their integrity at the temperature of the refrigerant used as well as the temperatures and pressures which could result if refrigeration were lost.
- Genetically modified organisms
GM Organisms or Micro-organisms should be packaged following the same principles as for Category B Infectious substances above.
- Purchasing packaging
Suitable packaging for the transport of dangerous goods can be purchased from the following suppliers:
Marking and Labelling
Marking and labelling is very important. See notes below.
- Category A infectious substances
You must consult the UCL Dangerous Goods Safety Advisor for advice before packaging and transporting Category A Infectious Substances as specific legislative requirements and security controls apply.
- Category B infectious substances
- The mark shown to the right (UN 3373) must be displayed on an external surface of the outer packaging. Each side of the diamond should have a length of at least 5 cm; the width of the line shall be at least 2 mm and the letters and numbers shall be at least 6 mm high.

The proper shipping name "Biological Substance, Category B" in letters at least 6 mm high must also be marked on the outer package adjacent to the diamond-shaped mark.
The name and address of the shipper (person sending the package) and of the consignee (person receiving the package) must be provided on each package. The name, address and telephone number of a person responsible must be provided on a written document (such as an air waybill, which accompanies the transport) or on the package itself.
- Dry ice
- The package (the outer packaging or the overpack) shall be labelled with a Class 9 'Miscellaneous Dangerous Goods' label along with the words "UN 1845" and "Carbon dioxide, solid" or "Dry ice".
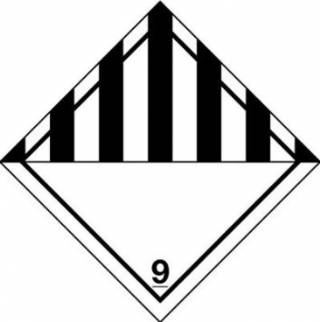
The net weight of the dry ice (solid carbon dioxide) must also be marked, alongside the description "UN 1845 Dry Ice". If packages are placed in an overpack, the overpack must be marked on the outside with the total net quantity of dry ice in the overpack.
- Genetically modified organisms
- The diamond-shaped mark shown top right must be displayed on an external surface of the outer packaging, but "UN 3245" must but used in place of "UN 3373" in the diamond. Each side of the diamond should have a length of at least 5 cm; the width of the line shall be at least 2 mm and the letters and numbers shall be at least 6 mm high.
The name and address of the shipper (person sending the package) and of the consignee (person receiving the package) must be provided on each package.
For all of the categories above, packages must also be labelled. If a liquid, with orientation arrows on two opposite vertical sides of the outer package (these are not required if transporting solids).
In all cases, make sure to remove any other labels or symbols that may be on the box - particularly if the box has the names of other organisations or other addresses.
Documentation
Documentation requirements will vary depending on the precise nature and quantity of the substance being transported and the mode of transport used, however, the guidance below is included to provide some general principles which can be followed, while you await detailed advice.
- Transport by road
If classed as Limited Quantities, no documentation is required. The courier you use may require documentation to accompany the consignment beyond that required by regulations.
- Transport by air
A Declaration for Dangerous Goods must be completed for all consignments, except Category B Biological substances, Genetically Modified Organisms or Micro-organisms and Carbon dioxide, solid (dry ice) when used as a refrigerant other than for dangerous goods. An example of a form can be found at the following link:
Transport
- Do
- Contact someone in your department who has completed the 'Carriage of Diagnostic and Infectious Substances by Air' training to obtain advice.
- Use approved couriers if transport is necessary. Details can be found at UCL mail and courier services.
- Make advance arrangements with the receiver of the goods to agree delivery Investigate the need for permits, licences or other customs approval if moving substances from one country to another, particularly if outside the EU.
- Don't
- Transport dangerous goods in your own car or a taxi as the vehicle is unlikely to be insured for this activity Transport dangerous goods on the Tube, buses or other public transport - it may attract unwanted attention or Police action.
- Carry dangerous goods, including infectious/biological material in your own luggage on aircraft - It is prohibited to carry goods on your person, in carry-on or checked baggage aboard aircraft.
- For general information on what substances or articles can and cannot be sent by Royal Mail (regular post), please read the following document for information.
- Emergencies
In the event of spillage during transport of dangerous goods
- Isolate spill or leak area immediately in all directions - it may be necessary to evacuate the room and not re-enter for a minimum of 15 minutes. This will allow any aerosols to settle
- Keep unauthorised personnel away
- Obtain identity of substance involved if possible
- Do not touch or walk through spilt material
- Do not touch damaged containers or spilt material unless wearing appropriate protective clothing
- Damaged packages containing dry ice (solid carbon dioxide) as a refrigerant may produce water or frost from condensation of air. Do not touch this liquid as it could be contaminated by the contents of the package
- Wear Personal Protective Equipment (PPE) appropriate to the material spilt
- If a liquid - absorb spillage with sand or other non-combustible absorbent material while avoiding direct contact with the substance. You may also need to use a disinfectant to inactivate the substance
- If a solid - sweep up the solid using a dustpan and brush being careful not to create dust - if necessary damp down the solid before sweeping
- Wipe down any potentially contaminated surfaces with disinfectant solution and place used wipes inside an appropriate waste bag
- Carefully remove disposable PPE and also place inside an appropriate waste bag
- Dispose of the waste by appropriate means (either direct to incineration or to autoclave)
- Notify UCL Safety Services by raising an incident report
In the event of theft or damage during transport not involving a spillage
- Contact the courier or other company involved in transport to notify them and request an investigation.
- Notify UCL Safety Services by raising an incident.
If the leaking contents accidentally come into contact with skin or clothes
- Thoroughly wash off the body with plenty of water.
- Remove contaminated clothing.
- Keep hands away from eyes nose and mouth.
- If the contents are identified as potentially harmful, staff who have come into contact may be advised to visit UCL Workplace Health or A&E.
Competence
Shippers (those sending a package) must be competent to ensure packaging and labelling are correct and that any documentation is completed correctly. All groups or departments that routinely transport biological material must have access to a competent person to advise on packaging, labelling and documentation.
Safety Services runs an accredited training course on the Carriage of Diagnostic and Infectious Substances by Air which outlines how biological materials must be classified, prepared, packaged and labelled for safe transport by air.
> Access the Carriage of Diagnostic and Infectious Substances by Air course on MyLearning.
Last updated: Monday, August 3, 2020
 Close
Close


