Up to date, we have collected a very wide range of clinical and self-reported information on our participants.
| Phase | Dates | Type | Participation | Response rate (alive) | Response rate (eligible*) |
|---|---|---|---|---|---|
| 1 | 1985-1988 | Questionnaire 1 & Clinic | 10,308 | -reference- | -reference- |
| 2 | 1989-1990 | Questionnaire 2 | 8,132 | 79.3% | 79.3% |
| 3 | 1991-1994 | Questionnaire 3 & Clinic | 8,815 | 86.6% | 86.6% |
| 4 | 1995-1996 | Questionnaire 4 | 8,628 | 86.6% | 92.4% |
| 5 | 1997-1999 | Questionnaire 5 & Clinic | 7,870 | 78.7% | 86.3% |
| 6 | 2001 | Questionnaire 6 | 7,355 | 74.4% | 82.5% |
| 7 | 2002-2004 | Questionnaire 7 & Clinic | 6,967 | 71.6% | 82.2% |
| 8 | 2006 | Questionnaire 8 | 7,173 | 75.2% | 87.2% |
| 9 | 2007-2009 | Questionnaire 9 & Clinic | 6,761 | 72.3% | 84.5% |
| 10 (**) | 2011 | Questionnaire 10 & Clinic | 277 | n/a | n/a |
| 11 | 2012-2013 | Questionnaire 11 & Clinic | 6,318 | 70.9% | 84.1% |
| 12 | 2015-2016 | Questionnaire 12 & Clinic | 5632 | 66.6% | 80.2% |
| 13 | 2019-2022 | Questionnaire 13 & Clinic | 4307 | 56.4% | 70.6% |
* Eligible: participants alive and not withdrawn from the study.
**Phase 10 was a pilot carried out in February and March 2011. A small group of Stress and Health participants were selected randomly and invited to attend our Phase 10 clinic. This enabled us to successfully pilot new measures for mental well-being introduced at Phase 11.
The study is approved by the London-Harrow Research Ethics Committee and the Scotland Research Ethics Committee. All participants who had clinical examination were asked to give written informed consent.
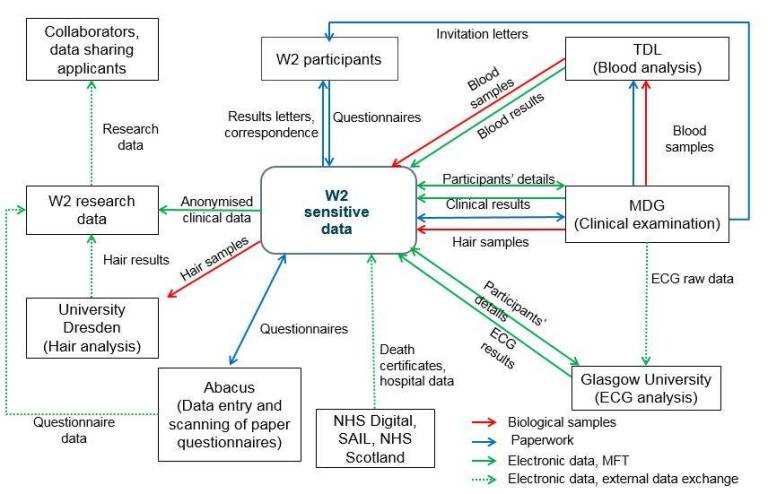
Documentation
Data Processing
Please check our Frequently Asked Questions (FAQs) on data processing.
 Close
Close

