Cell ‘skeletons’ built with strands of DNA
12 June 2023
The tiny tubes and thread-like structures that give cells their shape and help determine their function have been artificially re-created using strands of DNA in a study led by UCL researchers.
The research, published in Nature Communications, represents a key step towards synthetic “smart cells” that could be used to sense diseases, deliver drugs or repair damaged cells inside the body.
Cells, about a thousandth of a millimetre in size, are the fundamental units of all life. They contain “skeletons” made of proteins that fulfil a number of functions, such as providing structural support, helping the cell move around, and transporting materials within the cell.
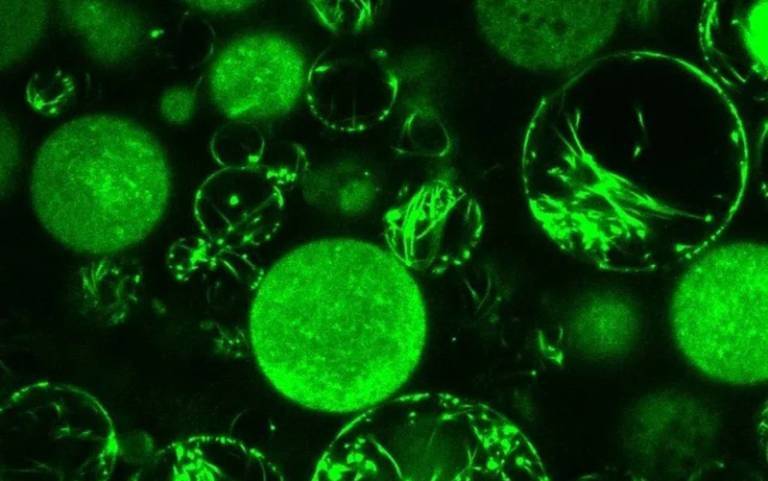
Re-creating these tubes and threads using proteins is challenging, so the researchers used strands of DNA as building blocks, and were able to precisely customise the structures’ dimensions (from about 20 to 400 nanometres thick) and stiffness (from flexible to ultra-rigid).
These tubes and threads were integrated inside cell-like sacs as well as coated on to the sacs’ exterior – functioning as a cytoskeleton (inside the cell) or exoskeleton (outside the cell). Most bacteria have what can be described as an exoskeleton, whereas plants, animals and other multicellular organisms have a cytoskeleton.
The tubes and threads were found to stabilise the sacs (vesicles), reducing the chance of them rupturing, in a similar way to how these skeletal structures work in real cells.
The team was also able to control the exact location of the tubes and fibers in real-time while they were inside the vesicles by attaching magnetic nanoparticles to the structures using an external magnet.
Senior author Dr Jonathan Burns (UCL Chemistry) said: “DNA is used by nature to store genetic information but it can also be used as a building material to construct nanostructures. We reprogrammed DNA to form synthetic skeleton structures inside model cells and tissues.
“We believe this work can help to unlock future smart cells able to sense diseases, repair damaged cells by fusing with them, and deliver drugs in a more targeted way – for instance, by carrying a drug or antibiotic and releasing it exactly where it is needed in the body.
“This initial study gave promising signs that these protocells may have limited toxicity for humans and the next step is to move from the laboratory to animals to investigate further how these protocells interact with living tissue.
“We need to ensure they are stable in the body and able to circulate the blood stream - then we can adapt them to target cancers or pathogenic bacteria.”
In addition, the researchers showed how their protocells could combine to form something analogous to living tissue. They placed the protocells in a solution of water and sugar. The cells sank (as they were heavier than the solution) and the evaporation of the water induced a swirling current that pushed the cells together. The team were able to bind these cells more tightly together by placing nanotubes or fibers on the exterior of the cells (giving the cells a “hairy” appearance). This caused the cells to lose their spherical shape and form a honeycomb pattern.
First author Dr Nishkantha Arulkumaran (UCL Division of Medicine) said: “Many of the building blocks we used in these protocells occur naturally in the body and we hope to extend this so that we can create smart cells entirely from substances such as lipids that are ordinarily found in our bodies and therefore can easily be broken down and either recycled or discarded.”
The researchers created the tubes and threads by placing strands of DNA in a solution of magnesium chloride, which is heated and then cooled at a fixed rate of half a degree a minute. This triggered the DNA to self-assemble into an ordered structure. By varying the magnesium concentration of the solution, the researchers were able to determine the dimensions and stiffness of the structure.
To form the vesicles and to get the nanostructures inside these vesicles, the team used an established method, placing the nanostructures in a solution of water and sugar (sucrose) and adding this to a layer of oil and lipids and another layer of glucose.
Spinning (centrifuging) this combination of substances resulted in droplets of oil with a membrane composed of a double layer of lipids, mimicking the membrane that separates cells from the outside world, with the nanostructures migrating inside these droplets.
The research was supported by the Rosetrees Trust and the Medical Research Council.
Links
- Research paper in Nature Communications
- DNA Fibers – research team’s website
- Dr Jonathan Burns’s academic profile
- Dr Nishkantha Arulkumaran’s academic profile
- UCL Chemistry
- UCL Mathematical & Physical Sciences
- UCL Division of Medicine
- UCL Medical Sciences
Image
- Confocal laser scanning microscopy image of the DNA fibers and nanotubes inside the protocells.
Media contact
Mark Greaves
T: +44 (0)7990 675947
E: m.greaves [at] ucl.ac.uk
 Close
Close

