Diseases
The group focuses on motor neuron diseases (MND), specifically on amyotrophic lateral sclerosis (ALS) and spinal bulbar muscular atrophy (SBMA).
Amyotrophic lateral sclerosis
ALS is the most frequent form of MND, and is often referred to as “motor neuron disease”. ALS is characterised pathologically by the progressive degeneration and death of both upper and lower MNs. It is the most aggressive form of adult-onset MND and causes death on average three years after onset mostly due to the involvement of the respiratory MNs, which leads to respiratory failure. There is an MND service at the National Hospital for Neurology and Neurosurgery.
Genetic research has identified causative mutations in a small proportion of patients. These findings have been invaluable to research as they have allowed the generation of disease models. We currently are focusing on two genes: TARDBP and FUS. Both genes encode for hnRNPs and are involved in multiple steps of RNA metabolism, including splicing, miRNA biogenesis, stress granule formation and RNA transport.
Spinal bulbar muscular atrophy
SBMA, also referred to as Kennedy’s Disease, is a slowly progressive form of MND that involves the degeneration of the LMNs
SBMA is caused by a mutation in the androgen receptor gene (AR) which is located on the X chromosome. Whilst normal individuals carry 9-36 CAG repeats in the AR exon 1, SBMA patients carry between 38 and 62 repeats.
SBMA is characterized by the loss of LMNs and a myopathy which lead to a generally slowly progressive weakness.
We run the UK National Registry for Kennedy’s Disease and there is a dedicated clinic for Kennedy’s Disease at the National Hospital for Neurology and Neurosurgery.
Projects
We use mouse models and in vitro systems to understand basic pathogenic mechanisms and are investigating alterations in RNA metabolism, with a focus on axonal RNA.
We are also carrying out clinical research with the goal of developing biomarkers and better outcome measures for disease progression, which are vital for future clinical trials.
Identifying motor neuron specific RNA in vivo, in ALS
(with Lizzy Fisher, UCL ION)
Aim: to identify cell-specific transcription changes occurring in mice models of ALS at different disease stages.
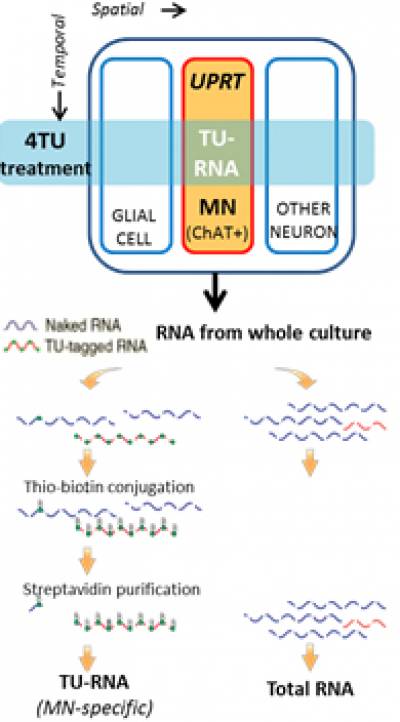
Our collaborator, Chris Q Doe, has developed a technique, TU-tagging, which through the cell-specific expression of an enzyme (UPRT) and the treatment with a drug (4TU), allows incorporation of thio-uridine into RNA in specific cell types. Thio-uridine allows to biotinylate and then pull-down the specific RNA. The Doe laboratory has successfully shown that by using this technique, it is possible to isolate and sequence RNA species from neuronal subtypes in drosophila and in mouse.
We are using this tagging technique to isolate and identify RNA specifically from MN cell bodies, dendrites and axons.
The role of axonal RNA alterations in ALS
Aim: to address the hypothesis that early changes in axonal biology, and particularly in RNA localisation and axonal signalling, play a role in ALS.
Due to their unique morphology, axons represent >95% of MN mass and play a crucial role in neuronal function. Crucially, they are affected very early in the course of ALS.
The delivery of RNAs to axons is important for their development, maintenance and response to injury.
Microfluidic chambers (developed in the Schiavo Lab) allow the physical separation of motor axons from cell bodies. We use this system to study the RNA content and dynamics in motor axons.
We are using a novel mouse model of ALS carrying mutations in FUS (Lizzy Fisher and Anny Devoy) as a paradigm of disease.
Our investigation techniques include FISH, RNAseq, live axonal transport imaging.
Defining TDP43 dependant RNA changes
(with Abraham Acevedo-Arozena)
Aim: to identify changes in RNA metabolism due to TDP43 loss and gain of function in mammalian CNS.
Neuronal TDP43 cytoplasmic inclusions are present in >98% of ALS post-mortem cases and represent a major hallmark of the disease. They are intriguingly accompanied by nuclear depletion of TDP43, raising the question whether there is a nuclear loss of function in RNA processing.
We are currently using two novel models carrying point mutations in TDP43 which cause widespread changes in RNA metabolism, to better understand the biology of TDP43 and its function in the CNS.
We also use muscle biopsy material, where TDP43 shows pathological aggregation, to study RNA profiles occurring in patients.
The role of miRNAs in ALS and their use as a biomarker of disease progression
(Collaborators: Eran Hornstein and Adriano Chio’)
Aim: to comprehensively address the potential of plasma miRNA as disease biomarkers, to further use mouse models to gain an understanding of the biological processes that determine plasma miRNA signatures, and to investigate whether a pharmacological intervention affecting miRNA processing can impact the course of ALS.
The potential of serum/plasma miRNAs as an ALS biomarker has been highlighted by several studies. Unlike mRNAs, miRNAs are stable in serum making them an accessible and reliable measurement in patients.
In contrast to other canonical biomarkers (e.g. serum proteins) that each require a specific assay, thousands of miRNA transcripts can be probed with one single assay, allowing the identification of multiple complex signatures, which can be integrated in a multi-modal analysis with other biomarkers, clinical data and imaging, to identify disease subgroups. Several studies have investigated serum miRNAs as potential biomarkers in ALS and have identified changes (De Felice et al., 2014; Freischmidt et al., 2013, 2014; Toivonen et al., 2014).
We use miRNAseq to investigate the levels of serum/plasma miRNA, and apply this to a very well curated longitudinal collection opf patient samples and a range of mouse models.
Skeletal muscle MRI as a biomarker for ALS and SBMA
(with Tarek Yousry and John Thornton)
Aim: to develop a pipeline which will allow us to conduct small and effective clinical trials. We will combine novel biomarkers to stratify patients in homogeneous groups and validate the use of skeletal muscle MRI as a sensitive progression marker for ALS and SBMA.
Although many clinical trials have been conducted in ALS, testing more than 100 compounds, all have failed to identify any effective therapy, except for Riluzole, which has only mild disease-modifying effects. Nevertheless, it is possible that some of these compounds may have a beneficial effect in ALS subtypes, but these may have been missed due to two important limitations to ALS trials: 1) the use of very crude outcome measures and 2) the great variability in ALS clinical progression.
In order to overcome these limitations, there is now a consensus in the ALS community that in order to improve ALS clinical trials there is a critical need for:
- disease stratification, to identify more homogeneous patient groups;
- development of more sensitive outcome measures, to better assess disease progression and modifications of disease course.
The MRC Centre for Neuromuscular Disorders has recently developed protocols to use skeletal muscle quantitative MRI as a sensitive measure of clinical progression in a number of neuromuscular disorders including hereditary neuropathies and adult myopathies (Morrow J et al., Lancet Neurol 2015).
We have now adapted these protocols to study muscle groups in upper and lower limbs and the bulbar region, and are currently validating them in ALS and SBMA patients.
We are currently funded by:
UK Medical Research Council
Motor Neurone Disease Association
UCLH NIHR Biomedical Research Centre
Kennedy’s Disease UK
The Rosetrees Foundation
People
Nicol Birsa, PhD – Postdoc
Cristian Bodo, PhD – Lab technician and manager
Agnieszka Gromadzka, PhD – Postdoc
Jack Humphrey – PhD student (with Vincent Plagnol)
Uros Klickovic – MRes Student
Prasanth Sivakumar – PhD Student
Luca Zampedri – Research Nurse
Pietro Fratta
Collaborators
Gipi Schiavo, UCL Institute of Neurology
Linda Greensmith, UCL Institute of Neurology
Lizzy Fisher, UCL Institute of Neurology
Vincent Plagnol, UCL Genetics Institute
Adrian Isaacs, UCL Institute of Neurology
Abraham Acevedo-Arozena,
Andrea Malaspina, Queen Mary University
Eran Hornstein, Weizmann Institute
Bryan Traynor, NIH
Adriano Chio’, University of Turin
Gianni Soraru’, University of Padua
 Close
Close

