The science behind the research
The IMD has a branch of research exploring the use of novel, uniquely structured anode materials for use in lithium ion batteries (LIBs).
When compared to nickel type batteries (Ni-Cd, Ni-MN) or lead acid batteries, the LIB is characterised by its high energy/power density, low self-discharge, long lifespan and by being lightweight.
It is believed these anode materials will improve the capacity and retention ability of the LIB - factors that are currently limiting performance in silicon LIBs - and will lead to the development of a rechargeable LIB, with efficient out-performance.
Potential applications
When a rechargeable LIB with efficient out-performance is discovered it will impact all industries where batteries are used, including;
- Portable electronics
- Electric vehicles (EV)
- Energy storage system (ESS), i.e. for clean energy
And these industries are growing and finding new applications almost daily! One such example, in case of ESS, is that a LIB system can be set up with solar panels deserts to provide clean energy across the globe.
The challenge
The problem however is that during repasted cycles the material shows a drastic volume expansion which causes the anode to crack and pulverize.
The challenge is to develop a material which overcomes these problems of poor life span, low stability and drastic capacity drop demonstrated by current LIBs.
The novel uniquely structured anode materials that have been selected by the IMD have the potential to significantly improve LIB performance due to their stronger mechanical stability, as well as the enhanced capacity.
| Fig 1. Schematic of a Lithium Ion Battery device |
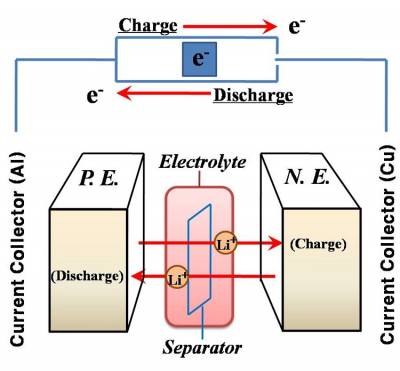 |
Ongoing research
| Fig 2. Nyquist Plot showing Impedance Data |
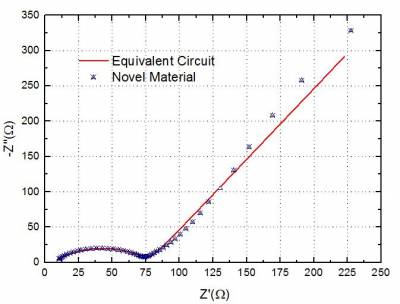 |
| Fig 3. Rate capability and cycling performance of oxygenally reduced graphene oxide (Or-GO) and thermally reduced graphene oxide(TrGO) electrode at current densities from 10 mA g−1 to 80 mA g−1 |
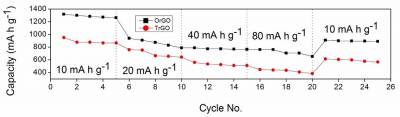 |
| Fig 4. Cycling performance and CE of OrGO and TrGO electrodes at a current density of 80 mA g−1 for 30 cycles |
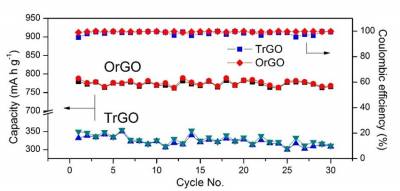 |
Recent Publications
- Choi, D. W., & Choy, K. L. (2020). Spider silk binder for Si-based anode in lithium-ion batteries. Materials & Design, 191, 108669. doi:10.1016/j.matdes.2020.108669
- Zhang, Z., Gonzalez, A. R., & Choy, K. L. (2019). Boron Nitride Enhanced Garnet-Type (Li6.25Al0.25La3Zr2O12) Ceramic Electrolyte for an All-Solid-State Lithium-Ion Battery. ACS Applied Energy Materials, 2, (10), 7438–7448. doi:10.1021/acsaem.9b01431
- Zhang, Z., Antonio, R. G., & Choy, K. L. (2019). Boron nitride enhanced polymer/salt hybrid electrolytes for all-solid-state lithium ion batteries. Journal of Power Sources, 435, 226736. doi:10.1016/j.jpowsour.2019.226736
- Choi, D., & Choy, K. (2017). Electrochemical Effect of Various Si/Zr Molar Ratios for Anode Materials in Lithium-ion Batteries. Dalton Transactions, 46, 14226-14233. doi:10.1039/C7DT01120B
- Choi, D., & Choy, K. L. (2016). Novel Nanostructured SiO2/ZrO2 Based Electrodes with Enhanced Electrochemical Performance for Lithium-ion Batteries. Electrochimica Acta, 218, 47-53. doi:10.1016/j.electacta.2016.08.116
 Close
Close

