It is predicted that one in two people in the UK will be diagnosed with cancer in their lifetime.
Our team of scientists at UCL have designed a virtual modelling technique which can create highly detailed 3D models of individual cancerous tumours and simulate the delivery of drugs into them.
This helps us to predict the effectiveness of cancer drugs and has significant potential for the development of personalised cancer therapies.
The problem
With an increasingly ageing population, one in two people in the UK will be diagnosed with cancer in their lifetime. As a result, cancer treatment is expected to cost the NHS £13.2 billion by 2021. There is an urgent need to develop novel and effective therapies to cope with these demands.
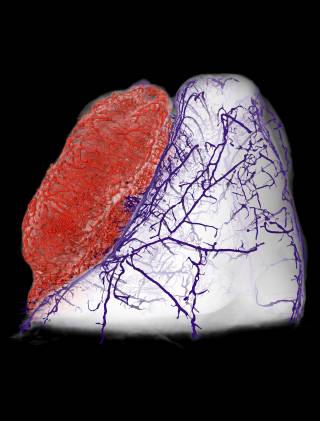
However, it is incredibly hard to predict whether therapies such as drugs will target a cancerous tumour effectively. Tumours’ structures vary widely, with dense and chaotically-formed networks of blood vessels that differ between each individual tumour and even between different regions of the same tumour.
This complex tumour architecture makes the delivery of therapeutic drugs particularly difficult and means that it is hard to predict the uptake of a drug by diseased tissue and its subsequent distribution. This can result in suboptimal dosing and a host of adverse side-effects, including increased resistance to treatment through exposure.
Current clinical methodology using medical imaging has so far proved unable to make successful predictions about the uptake and efficacy of cancer drugs.
The solution
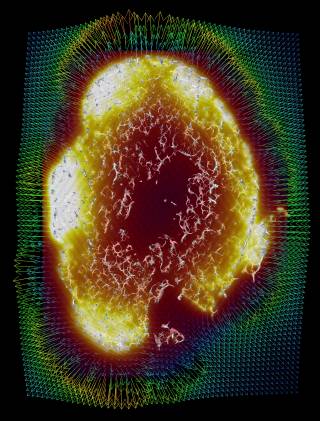
Our researchers at UCL have combined advanced imaging techniques with mathematical modelling, creating virtual simulations able to investigate how drugs are distributed once injected and help predict tumour response.
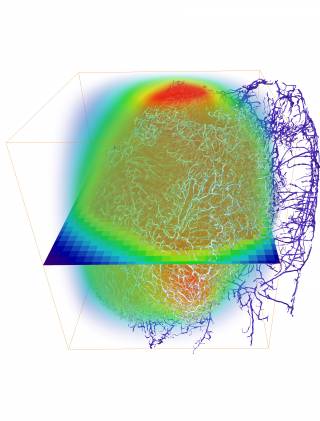
The framework, named REANIMATE(REAlistic Numerical Image-based Modelling of biologicAl Tissue substratEs), reconstructs tumours by integrating high-resolution imaging and computational modelling. This allowed us to run detailed experiments and study the transport of blood, biological fluids and drugs, and their complex interactions with tissue.
The impact
This new framework has a vast potential impact in helping develop new cancer drugs and providing a cost-effective way to test their efficacy before going to human trials.
It advances the move towards truly personalised medicine, with the potential that one day clinicians may be able to predetermine the most effective therapeutic plan for each patient’s unique tumour make-up.
The science
Our REANIMATE framework enables us to visualise and interact with large, 3D, virtual models of tumour tissue samples and treat them as living specimens. We acquired high-resolution images of surgically-resected tumours and used mathematical modelling to run detailed computational experiments. These experiments generate new insights into how individual tumours react to specific treatments.
The work combines ex vivo and in vivo imaging with mathematical modelling to virtually reconstruct cancer tumours for these simulations. This integration is a novel approach that provides an entirely new framework for therapy prediction in tumours.
A recent study, published in Nature Biomedical Engineering,showed how we used optical imaging of extracted tumour tissue that had been rendered transparent using a cocktail of chemical treatments. These can show fine detail such as blood vessel networks and cell nuclei, which can be seen across entire organs at very high resolution by using fluorescently-labelled probes that bind to specific structures.
The team
Our team is highly interdisciplinary and our research would not be possible without the combined input of mathematicians, cancer biologists, clinicians, imaging specialists and engineers. The research was led by Dr Paul Sweeney, Dr Simon Walker-Samuel and Dr Rebecca Shipley, with UCL Division of Medicine, UCL Mechanical Engineering and UCL Institute for Healthcare Engineering, in close collaboration with many other colleagues and with the support of the Rosetrees Trust and the Wellcome Trust.
 Close
Close