Helping to identify patients with ALK-positive lung cancers
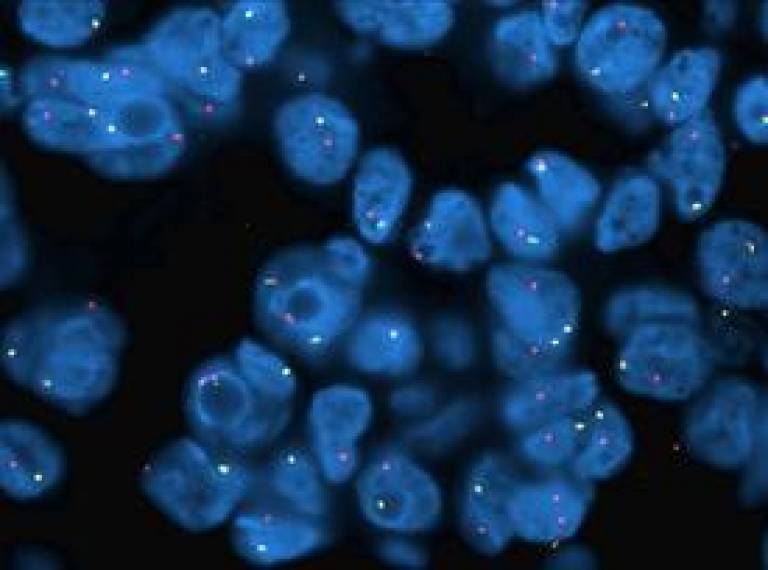
Today immunocytochemistry (IHC) is routinely used for the diagnoses and prognosis of a number of diseases, including cancers.
27 February 2015
This technique involves staining tissue sections using antibodies which specifically bind to particular cell proteins. The antibodies are then detected using secondary reagents which are coupled to enzymes. When the enzymes react with their substrates, colour develops allowing the identification of individual cells within a tissue that carry the protein of interest.
However, correct tumour classification, interpretation and subsequent selection of patients for specific treatment/s, very much depends on the laboratory employing the correct IHC methodology.
Pinpoint the production of a protein, pinpoint the therapy
This project is one where UCL Consultants (UCLC) managed the contractual and financial arrangements for Novartis to provide an education grant to Dr Merdol Ibrahim at the United Kingdom National External Quality Assessment Service for Immunocytochemistry and In-Situ Hybridisation (ICC & ISH), which is an independent organisation under the umbrella of the UCL Department of Pathology.
Working with Dr Chandra Ratnam, Senior Medical Advisor (Oncology) at Novartis Pharmaceuticals UK Limited, Dr Ibrahim's project aims to establish an External Quality Assessment (EQA) module for ALK testing specifically in non-small cell lung carcinoma. This is a crucial area of study because an ALK positive lung cancer is a malignant tumour where cells contain an abnormal configuration of DNA such that the echinoderm microtubule-associated protein-like 4 (EML-4) gene is fused to the anaplastic lymphoma kinase (ALK) gene. This abnormality leads to the over production of ALK proteins which promotes and maintains the malignant behaviour of the cancer cells.
Currently, existing therapies for lung cancer sufferers have limited benefit due to their non-specific targeted approach. But by accurately identifying underlying molecular or mutational changes certain sub-groups of patients may be eligible candidates for more precisely targeted therapies. Thus those patients may have a greater chance of survival.
How does an External Quality Assessment (EQA) help
By objectively and frequently monitoring a laboratory's' performance using test samples of known protein levels, an EQA provides in-depth information as to whether clinical laboratories can correctly identify mutations using their chosen methodology.
Not only does this give laboratories reassurance and confidence in their chosen methodology to correctly identify patients who may be put forward or are eligible for a specific therapeutic treatment regime. UK NEQAS ICC & ISH also establishes the best means to test laboratories by selecting the most appropriate and clinically relevant samples to use for quality control.
By focusing on lung cancer and enhancing NEQAS's current regime of offering assessments in seven different immunocytochemistry modules at approximately three-monthly intervals, this year-long project provides laboratories with guidance to improve and standardise their IHC methodologies to correctly identify ALK-positive lung cancer patients who may respond to specific cancer drugs. This could result in improving overall survival rates.
Ensuring contractual obligations, milestones and funding payments are met
Due to the nature of the work of UK NEQAS ICC & ISH, which was not strictly research-based, UCL Consultants were the ideal partners to look after the contractual and financial arrangements of the Novartis grant.
As Dr Merdol Ibrahim explained, 'UCL Consultants not only advised on contractual and financial terms for UK NEQAS - negotiating and implementing changes that were requested - but as the project moves forward, they will continue to ensure the contractual obligations for the project are appropriate and that funding is transferred to UK NEQAS after each project milestone has been met.'
 Close
Close

