| Introduction |
| Dinoflagellates are protists which have been classified using both the International Code of Botanical Nomenclature (ICBN) and the International Code of Zoological Nomenclature (ICZN), approximately half living dinoflagellate species are autotrophs possessing chloroplasts and half are non-photosynthesising heterotrophs. It is now widely accepted that the ICBN should be used for their classification. Dinoflagellates and their cysts belong to the Division Pyrrhophyta (literally "fire plants"), Class Dinophycaea, the related Class Ebriophyceae (also in the Division Pyrrhophyta) includes the ebridians which have internal siliceous skeletons, are extant and have a fossil record beginning in the Palaeocene. An important point to remember about dinoflagellates is that the vast majority of the fossil record consists of cysts (dinocysts), and only 10% of living dinoflagellates are known to produce cysts. |
| Dinoflagellates possess two flagella, one (the transverse flagellum) may be contained in a groove-like structure around the equator of the organism (the cingulum), providing forward motion and spin to the dinoflagellate, the other (the longitudinal flagellum) trailing behind providing little propulsive force, mainly acting as a rudder. Another characteristic of the dinoflagellates is the wall composition and structure; early classification of the dinoflagellates was based on the presence (termed armoured) or absence (termed unarmoured) of a rigid outer cell covering (or theca). Evidence has since been found to suggest there is an intergradation between these types. The pattern (or tabulation) of armoured plates which form the theca of the so-called armoured forms is still a vital element of not only dinoflagellate classification but dinocyst classification as well. This is because the tabulation of a dinoflagellate may be reflected in the features of the cyst it produces (this is correctly referred to as paratabulation). |
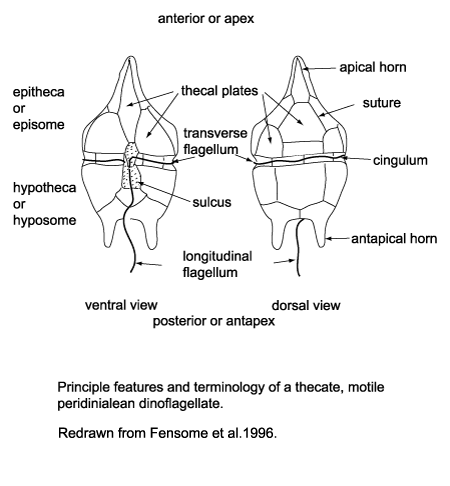 |
 |
| History of Study |
| The first modern dinoflagellate was described by Baker in 1753, the first species was formally named by Muller in 1773. The first fossil forms were described by Ehrenberg in the 1830's from flint of Cretaceous age. In 1933, Wetzel established the genus Hystrichosphaera for the spiny spherical forms Ehrenberg had seen, giving rise to the general term hystrichosphere. In the early 1900's Kofoid produced a standardised description of dinoflagellate tabulation which is still in use today. In 1961 Evitt recognised that the openings on the walls of cysts correspond to the plates on the theca of dinoflagellates (whilst the cysts themselves do not have seperate plates), he called these openings archeopyles. Evitt also realised that the spines or processess seen on many cysts reflect the tabulation pattern of the original dinoflagellate from which the cyst emerged. In 1964 Evitt and Davidson attempted to associate dinoflagellates with their cysts In 1968 Wall and Dale documanted the excystment of the living dinoflagellate Gonyaulax from a Spiniferites-type cyst. It was not untill 1993 that Fensome and Taylor produced a paper with dinoflagellates and their cysts clearly related to each other. |
 |
| Range |
| Dinoflagellates are considered to be amongst the most primative of the eukaryotes. The earliest continuous record of dinocysts comes from mid Triassic age deposits found in Australia, while the very earliest recorded fossils with dinocyst affinities are from Silurian strata. It is thought that the earliest dinoflagellates may not have produced cysts, or they produced fossilisable cysts which are not recognised as such. It should be remembered that what are classified as acritarchs (common in Palaeozoic rocks) may actually be dinoflagellate cysts. Because of the complex multi-stage life cycle of modern dinoflagellates and their known ability to evolve from non-cyst forming strategies to cyst forming strategies, it is almost impossible to reproduce dinoflagellate/dinocyst evolutionary history. In terms of an overall trend of dinocyst species diversity there is a steep increase during the Jurassic and early Cretaceous, reaching a peak in the mid Cretaceous. A sharp decline occurred from the mid Cretaceous followed by an equally sharp increase reaching another peak during the Maastrichtian. From the Maastrichtian to the late Palaeocene diversity declined again followed by another increase untill the early Eocene. From then to the present diversity has steadily declined to a level perhaps one third that of the peaks reached during the Cretaceous. |
 |
 |
| Classification |
| In 1993 Fensome and Taylor linked dinoflagellates to their cysts emphasising the tabulation/paratabulation in their classification. Dinoflagellates are classified as Protists within the division Dinoflagellata, most of the members of this division are charcterised by having, during at least one part of their life cycle, a motile stage with two dissimilar flagella. Two subdivisions are recognised, of which the Dinokaryota possess a dinokaryon (the typical dinoflagellate nucleus) during at least part of their life cycle. Within the Dinokaryota the class Dinophyceae encompasses taxa with a permanent dinokaryon and contains all known fossils (though for the fossils, of course, the relationship is assessed by means other than the character of the nucleus). Within the Dinophyceae three subclasses are palaeontologicaly important, the Gymnodinophycidae, the Peridiniphycidae and the Dinophysiphycidae. Within the subclass Peridiniphycidae the orders Gonyaulacales and Peridiniales contain most of the known fossil dinoflagellates. |
 |
| Applications |
| Dinocyst biostratigraphy has been utilised very successfuly, particularly in situations where calcareous microfossils are not preserved or abundant, for instance in the clastic Mesozoic sediments of Alaska and the North Sea. However, there are few dinoflagellate biozonations applicable over large areas or time spans, first and last appearence datums are utilised to produce basin-wide biozonations which have proved stratigraphicaly valid even though they are not based on a "natural" systematic classification. Palaeobiological applications of dinocysts encounter the same problems as biostratigraphic applications. The most abundant fossil dinocyst assemblages are from neritic to upper bathyal environments. Several palaeoclimatic studies have recognised regionally restricted distributions or provinces. |
 |
| Biology |
| The majority of dinoflagellate species are marine, and together with coccolithophores and diatoms they make up the most important primary producers in the oceans. Dinoflagellates are also common in freshwater lakes, rivers and bogs and can occur in blooms of sufficient concentration to discolour the water, producing what are known as "red tides". Dinoflagellates are commonly studied during their motile, planktonic stage; cyst-forming dinoflagellates are known from all oceanic habitats but they dominate in shallow coastal waters where the cysts may seed oceanic populations. The distribution of dinocysts may follow patterns based on latitude, temperature, salinity, water depth and ocean circulation systems. |
| The cytoplasm of dinoflagellates contains typical eukaryotic organelles including; rough and smooth endoplasmic reticulum, Golgi apparatus, mitochondria, lipid and starch grains, food vacuoles etc. It may also contain one or several distinctive organelles which include: |
- The pusule which has been suggested may function in osmoregulation, waste disposal, flotation or nutrition.
- Light sensitive organelles, the eyespot and more complex ocellus.
- Chloroplasts bounded by three rather than the usual two membranes, which has led to suggestions that the chloroplasts in dinoflagellates were originally symbiotic algae.
- The nucleus which is large and contains a prominent nucleolus.
- During at least part of their life cycle most dinoflagellates have two dissimilar flagella, in some forms both appearing from the anterior end and from the ventral surface but in most cases one encircling it the other trailing it.
|
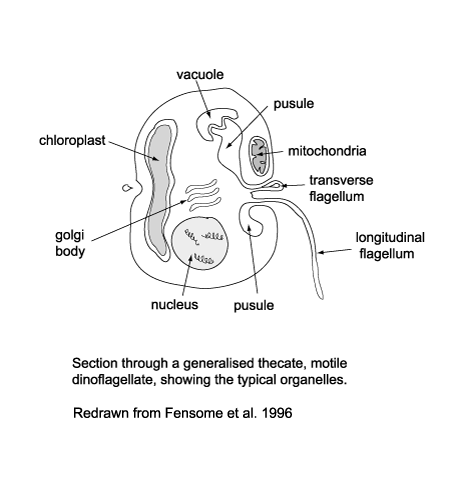
|
| Dinoflagellates exhibit a variety of feeding strategies, about half are autotrophic, since dinoflagellates have a slower generation time than diatoms they tend to follow diatom blooms. The ability of dinoflagellates to migrate up and down through the water column means they can take advantage of increased nutrient levels at greater depths during the night and return closer to the surface in order to photosynthesise during the day. They may also be better adapted to take advantage of nutrients at frontal systems due to their mobility. Heterotrophic dinoflagellates are known to feed on algae (including other dinoflagellates) eggs and larvae of other marine plankton, |
 |
| Lifecycle |
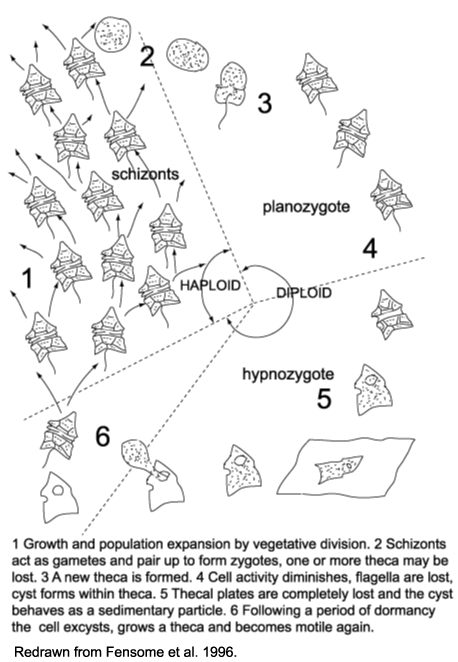
| The life cycle of dinoflagellates is multi-staged. Evitt (1985) recognised a six stage cycle in peridiniales dinoflagellates. |
- During periods favoured by rapid growth and population expansion, vegetative fission dominates yielding motile haploid schizonts.
- At an unknown trigger, the schizonts act as gametes and pair up and fuse to form diploid zygotes. One or more theca may be lost in the process.
- The diploid zygote constructs a new theca and resumes its motility as a planozygote.
- In several species the zygote theca becomes much thicker and considerably larger than the vegetative theca. Its outline becomes less regular, the protoplast becomes granular and reddish bodies are visible within it, the activity level decreases and after as many as fifteen days the flagella are lost, the cell is then termed a hypnozygote. The protoplast shrinks pulling away from the theca and eventually one or two membranes form a new cyst wall. The thecal plates then break apart or are decayed and the completed cyst is exposed.
- The hypnozygote or resting cyst then behaves as a sedimentary particle and settles to the sea floor.
- Following a period of obligate dormancy the protoplast excysts (typically through an archaeopyle), and the cycle closes as meiotic division again produces haploid, thecate, motile cells.
|
| In laboratories sexual reproduction may be induced by nutrient, temperature or light reduction. In nature sexual reproduction is known to occur in late summer and autumn and in the late stages of blooms. |
|
 |
| Preparation Techniques |
|
WARNING:
Please remember all preparation techniques require the use of hazardous materials and equipment and should only be carried out in properly equiped laboratories, wearing the correct safety clothing and under the supervision of qualified staff. In particular the preparation of organic walled microfossils requires the use of potentially lethal chemicals and procedures and should only be carried out by trained personnel. |
| Clays, silts, mudstones, sandstones, limestones and shales may first be broken down physically into less than five millemeter fragments. The sample is then placed in a plastic beaker in a fume cupboard and covered in hydrochloric acid to remove carbonates. The sample is then removed from the hydrochloric acid and placed in hydrofluoric acid to remove silicates. Once removed from the hydrofluoric acid the sample is checked in a wet mount to ascertain the next procedure required. If the sample is clean of mineral matter it can be oxidised either using nitric acid or Schultze's solution. If mineral matter is present boiling in hydrochloric acid may be required before oxidation. The sample is removed from the last acid treatment and can either be swirled to separate organic matter from any remaining mineral matter or heavy liquid separation can be used where a heavy liquid with specific gravity of 1.6-2.5 is added to the sample and centrifuged, the organic material floating to the surface. |
| Two techniques for washing the acid out of the samples can be used. The decanting method involves allowing the acid and sample mix to settle and then carefully decanting off the acid, topping up with distilled water and repeating untill the liquid is neutral, but this is a time-consuming method. The second method is to wash the acid and sample mix through an acid resistant sieve gauze of a mesh size suitable to retain the palynomorphs. |
| Staining of samples (with Safranin-0, for example) may be used depending on the natural colour remaining in the palynomorph.
Kerogen slides are often required and require a special preparation technique obviously without any oxidation or sieving to retain the total organic matter preserved in the sample. Once all acid treatment and separation processes are complete the sample may be mounted by strewing onto cover slips and allowing to dry. The inverted cover slip is then glued onto a slide using Norland Optical Adhesive or Petropoxy or similar proprietry glue of sufficient refractive index. |
 |
| Observation Techniques |
| Palynology slides are examined using transmitted light microscopes commonly with times forty dry and times one hundred oil immersion objective lenses. Accurate co-ordinates of individual specimens on a slide may be required and are often given using either the graduated scale on the traverseable slide table of a particular microscope or, preferably, by giving England Finder co-ordinates. An England Finder is a specially made slide which is divided into a grid of segments each one given an alpha-numeric reference. When a specimen is located on a slide the slide is carefully removed from the microscope and replaced by the England Finder and the co-ordinates marked down, as long as the orientation of the slide is also noted it is then possible to re-locate the specimen using the England Finder on any microscope. |
 |
| Images |
| The following images are of a representative selection of dinoflagellates aimed at giving a general overview of the different morphotypes. Each specimen is given a generic and, if possible, a species name followed by its age range, the site location from which the sample was obtained and its size in microns. LM (Light Microscope) SEM (Scanning Electron Microscope). Typical and selected marker species are illustrated from each main period of the geological column in which dinoflagellates occur. The images are divided into Cenozoic and Mesozoic forms, click on a link below or scroll down to each section. Click on an image to view a larger version. |
|
|
| Cenozoic |

|
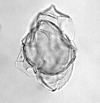
| Spiniferites bentori
(Wall and Dale)
|
| Pleistocene-Recent
|
| Kutai Basin, East Kalimantan, Indonesia |
| LM |
|
| Hystrichosphaeropsis obscura
Habib
|
| Aquitanian (Miocene)-Lower Pliocene
|
| Kutai Basin, East Kalimantan, Indonesia |
| LM |

|
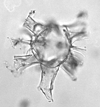
| Impagidinium patulum
Wall
|
| Burdigalian (Miocene)-Recent
|
| Kutai Basin, East Kalimantan, Indonesia |
| LM |
|
| Hystrichokolpona rigaudae
Deflandre and Cookson
|
| Ypresian (Eocene)-Pleistocene
|
| Kutai Basin, East Kalimantan, Indonesia |
| LM |

|
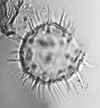
| Nematosphaeropsis labyrinthea
Ostenfeld
|
| ?Eocene-?Recent
|
| Kutai Basin, East Kalimantan, Indonesia |
| LM |
|
| Lingulodinium machaerophoram
(Deflandre and Cookson)
|
| Ypresian (Eocene)-Recent
|
| Kutai Basin, East Kalimantan, Indonesia |
| LM |

|
| Achomosphaera ramulifera
(Deflandre)
|
| ?Pliocene
|
| Kutai Basin, East Kalimantan, Indonesia |
| LM |

|
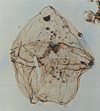
| Alisocysta margarita
Harland
|
| Thanetian (Palaeocene)
|
| Beryl Field, U.K North Sea |
| LM |
|
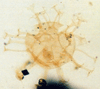
| Phelodinium magnificum
Stover and Evitt
|
| ?Palaeogene?
|
| Beryl Field, U.K North Sea |
| LM |
|
| Hystrichosphaeridium tubiferum
Davey and Williams
|
| Maastrichtian-Ypresian (Eocene)
|
| Beryl Field, U.K North Sea |
| LM |

|
| Deflandrea oebisfeldensis
Alberti
|
| Thanetian (Palaeocene)-Ypresian (Eocene)
|
| Beryl Field, U.K North Sea |
| LM |

|
| Cerodinium wardenense
Lentin and Williams
|
| Palaeogene?
|
| Beryl Field, U.K North Sea |
| LM |

|
| Thalassiphora pelagica
Benedek and Gocht
|
| Maastrichtian (Upper Cretaceous)-Chattian (Oligocene)
|
| Beryl Field, U.K North Sea |
| LM |

|
| Cordosphaeridium gracile
Davey and Williams
|
| Maastrichtian (Upper Cretaceous)-Rupelian (Oligocene)
|
| Beryl Field, U.K North Sea |
| LM |

|
| Cordosphaeridium inodes
Morgenroth
|
| ?Palaeogene?
|
| Beryl Field, U.K North Sea |
| LM |

|
| Operculodinium centrocarpum
Wall
|
| ?Palaeogene?
|
| Beryl Field, U.K North Sea |
| LM |

|
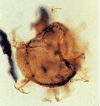
| Spniferites ramosus granosus
Lentin and Williams
|
| ?Palaeogene?
|
| Beryl Field, U.K North Sea |
| LM focus on parasutures |
|
| Spiniferites ramosus granosus
Lentin and Williams
|
| ?Palaeogene?
|
| Beryl Field, U.K North Sea |
| LM focus on granular periphragm |

|
| Spiniferites membranaceus
Sarjeant
|
| ?Palaeogene?
|
| Beryl Field, U.K North Sea |
| LM |

|
| Achomosphaera ramulifera
Evitt
|
| ?Palaeogene?
|
| Beryl Field, U.K North Sea |
| LM |

|
| Areoligera senonensis
Lejeune-Carpentier
|
| Maastrichtian (Upper Cretaceus)-Lutetian (Eocene)
|
| Beryl Field, U.K North Sea |
| LM |

|
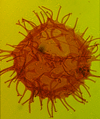
| Glaphyrocysta ordinata
Stover and Evitt
|
| Thanetian (Palaeocene)-Ypresian (Eocene)
|
| Beryl Field, U.K North Sea |
| LM |
|
| Apectodinium homomorphum
Lentin and Williams
|
| Thanetian (Palaeocene)-Bartonian (Eocene)
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |

|
| Operculodinium centrocarpum
Wall
|
| Paleocene?-Oligocene?
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |

|
| Kallosphaeridium orchiense
de Coninck
|
| Eocene
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |

|
| Lingulodinium machaerophorum
Wall
|
| Eocene-Recent
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |

|
| Adnatosphaeridium multispinosum
Williams and Downie
|
| Ypresian (Eocene)-Bartonian (Eocene)
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |

|
| Homotryblium pallidum
Eaton
|
| Eocene?-Miocene?
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |
 |
| Mesozoic |

|
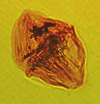
| Palaeohystrichophora infusorioides
Deflandre
|
| Albian-Maastrichtian (Upper Cretaceous)
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |
|
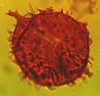
| Dinogymnium acuminatum
Evitt, Clarke and Verdier
|
| Maastrichtian (Upper Cretaceous)
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |
|
| Exochosphaeridium bifidum
Clarke et al
|
| Cenomanian-Campanian (upper Cretaceous)
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |

|
| Stephodinium coronatum
Deflandre
|
| Aptian-Turonian (middle Cretaceous)
|
| Borehole 2, Selborne , S.E England |
| LM |

|
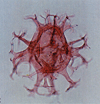
| Coronifera oceanica
Cookson and Eisenack
|
| Hauterivian-Maastrichtian (Cretaceous)
|
| Copt Point, S.E England |
| LM |
|
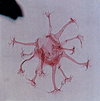
| Achomosphaera neptuni
Davey and Williams
|
| Ryazanian-Aptian (Lower Cretaceous)
|
| Copt Point, S.E England |
| LM |
|
| Oligosphaeridium complex
Davey and Williams
|
| Valanginian (Lower Cretaceous)-Palaeocene
|
| Copt Point, S.E England |
| LM |

|
| Ovoidinium verrucosum
Davey
|
| Upper Albian-Cenomanian (Cretaceous)
|
| Borehole 1, Selborne, S.E England |
| LM |

|
| Odontichitina operculata
Deflandre and Cookson
|
| Barremian-Maastrichtian (Cretaceous)
|
| Borehole 1, Selborne, S.E England |
| LM |

|
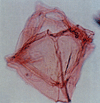
| Callaiosphaeridium asymmetricum
Davey and Williams
|
| Hauterivian-Early Campanian (Cretaceous)
|
| Borehole 1, Selborne, S.E England |
| LM |
|
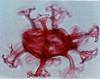
| Endoscrinium campanula
Vozzhennikova
|
| Ryazanian-Coniacian (Cretaceous)
|
| Borehole 2, Selborne, S.E England |
| LM |
|
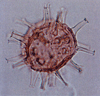
| Stiphrosphaeridium anthophorum
Lentin and Williams
|
| Hauterivian-Upper Albian (Cretaceous)
|
| Borehole 1, Selborne, S.E England |
| LM |
|
| Litosphaeridium arundum
Lucas-Clark
|
| Lower Albian-Cenomanian (Cretaceous)
|
| Copt Point, S.E England |
| LM |

|
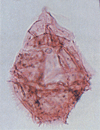
| Florentinia mantelli
Davey and Verdier
|
| Hauterivian-Turonian (Cretaceous)
|
| Copt Point, S.E England |
| LM |
|
| Gonyaulacysta cassidata
Sarjeant
|
| Early Aptian-Cenomanian (Cretaceous)
|
| Copt Point, S.E England |
| LM |

|
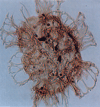
| Crobroperidinium edwardsii
Davey
|
| Barremian-Turonian (Cretaceous)
|
| Copt Point, S.E England |
| LM |
|
| Systematophora pencillata
Sarjeant
|
| Oxfordian-Upper Kimmeridgian (Upper Jurassic)
|
| Outer Moray Firth Basin, North Sea |
| LM |

|
| Gonyaulocysta jurassica
Poulsen
|
| Upper Bajocian-Lower Kimmeridgian (Jurassic)
|
| Outer Moray Firth Basin, North Sea |
| LM |

|
| Ctenidodinium ornatum
Deflandre
|
| Lower Callovian-Upper Oxfordian (Jurassic)
|
| Outer Moray Firth Basin, North Sea |
| LM |
 |
































































































