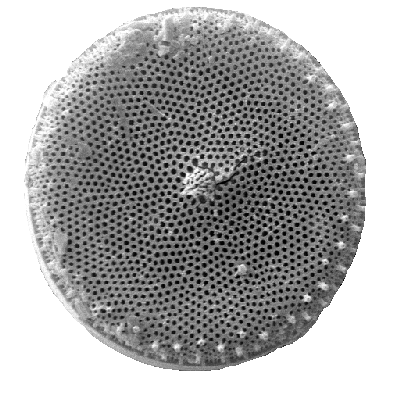
| Introduction |
| Diatoms are photosynthesising algae, they have a siliceous skeleton (frustule) and are found in almost every aquatic environment including fresh and marine waters, soils, in fact almost anywhere moist. They are non-motile, or capable of only limited movement along a substrate by secretion of mucilaginous material along a slit-like groove or channel called a raphe. Being autotrophic they are restricted to the photic zone (water depths down to about 200m depending on clarity). Both benthic and planktic forms exist. Diatoms are formally classified as belonging to the Division Chrysophyta, Class Bacillariophyceae. The Chrysophyta are algae which form endoplasmic cysts, store oils rather than starch, possess a bipartite cell wall and secrete silica at some stage of their life cycle. Diatoms are commonly between 20-200 microns in diameter or length, although sometimes they can be up to 2 millimeters long. The cell may be solitary or colonial (attached by mucous filaments or by bands into long chains). Diatoms may occur in such large numbers and be well preserved enough to form sediments composed almost entirely of diatom frustules (diatomites), these deposits are of economic benefit being used in filters, paints, toothpaste, and many other applications. |
| This site concentrates on marine diatoms since information on feshwater diatoms is already available at the
Environmental Change Research Centre at University College London
as well as many other web-sites. Please see the
links page
for more links to diatom resources available on the web. |
 |
| History of Study |
| Diatoms have been studied since the late eighteenth century, however the first real advances in the field came in the early nineteenth century when diatoms were popular with microscopists utilising the emerging improvements in microscope resolution. Several European workers produced hand illustrated monographs on diatoms in the late nineteenth century. Notable amongst these are the works of Cleve, Ehrenberg, Grunow, Schmidt and Van Heurck. In the early twentieth century fossil diatoms were first studied and, most famously, Hustedt (1927-66) produced a taxonomic and ecological study of diatoms which remains a key reference today. Perhaps the most complete treatment of diatoms is that of Round et al. (1990). |
 |
| Range |
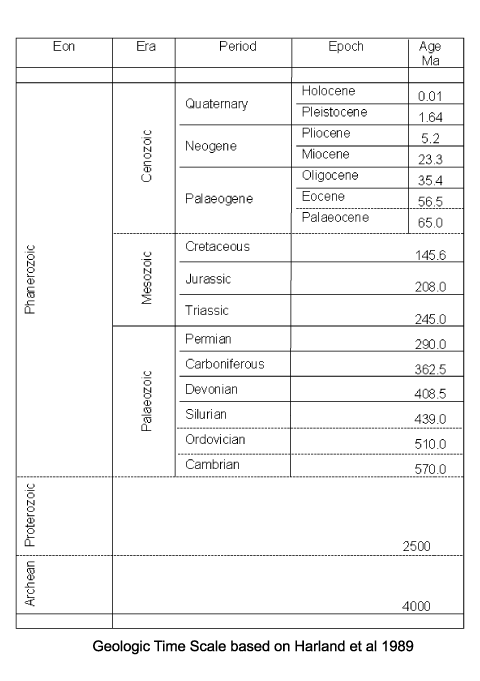
| First recorded occurrences of diatoms are from the Jurassic, however, these are uncertain and the earliest recorded well preserved diatoms are centric forms from the Aptian-Albian stages of the Cretaceous. Since these are quite diverse assemblages it is assumed diatoms have an earlier evolutionary history, perhaps lacking a relatively robust silica frustule. As with coccoliths, the earliest forms in the fossil record may reflect a biomineralisation event rather than an evolutionary appearance. The earliest araphid (lacking a raphe) pennate diatoms appear in the Late Cretaceous, and raphid pennates in the Middle Eocene. The earliest freshwater diatoms appear in the Palaeocene in Russia and the Late Eocene in North America. In a similar manner to Radiolaria, it has been noticed that there has been a gradual progression towards less heavily silicified frustules, probably as a result of increasing competition for a limited resource (silica). |
 |
 |
| Classification |
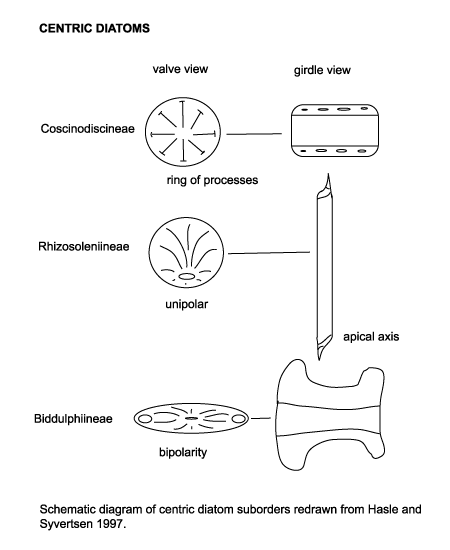
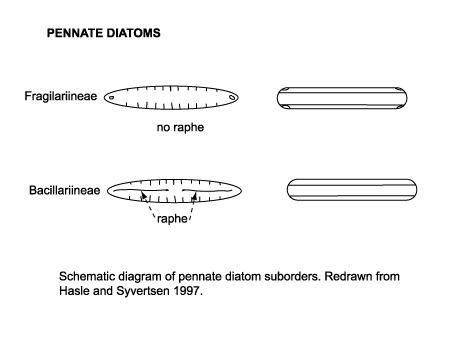
| Diatoms are divided into two Orders. The Centrales (now called the Biddulphiales) which have valve striae arranged basically in relation to a point, an annulus or a central areola and tend to appear radially symmetrical, and the Pennales (now called Bacillariales) which have valve striae arranged in relation to a line and tend to appear bilaterally symmetrical. The valve face of the diatom frustule is ornamented with pores (areolae), processes, spines, hyaline areas and other distinguishing features. It is these skeletal features which are used to classify and describe diatoms, which is an advantage in terms of palaeontology since the same features are used to define extant species as extinct ones. The classification system developed by Simonsen (1979) and further developed by Round et al. (1990) is currently the most commonly accepted. Diatoms commonly found in the marine plankton may be divided into the centric diatoms including three sub-orders based primarily on the shape of the cells, the polarity and the arrangement of the processes. These are the Coscinodiscineae, with a marginal ring of processes and no polarity to the symmetry, the Rhizosoleniineae with no marginal ring of processes and unipolar symmetry, and the Biddulphiineae with no marginal ring of processes and bipolar symmetry. The pennate diatoms are divided into two sub-orders, the Fragilariineae which do not posses a raphe (araphid) and the Bacillariineae which posses a raphe. |
 |
 |
 |
| Applications |
| The evolutionary history of diatoms has been punctuated by several floristic turnovers, these have been utilised to allow basin wide biostratigraphic correlations. Diatoms are also used extensively in palaeoenvironmental studies particularly in palaeoceanography. Dissolution of diatom frustules during descent through the water column, on the sediment surface and during diagenesis may seriously alter the preserved assemblage by preferentialy dissolving more lightly silicified forms. High alkalinity of pore waters and burial temperatures in excess of 50 degrees centigrade are also known to increase dissolution of silica. Incorporation into faecal pellets or muciligenous aggregations, rapid burial and the formation of heavily silicified resting spores tend to counteract these problems, however, in marine samples it is thought that only 1% to 5% of the living assemblage in surface plankton is represented in the death assemblage found on the sediment surface. Despite these problems diatoms are still a useful and to a certain extent under-utilised group in terms of biostratigraphy. |
| Living diatoms often have specific salinity, temperature and other environmental tolerences, this together with the fact that a high proportion of fossil genera and species are still extant, makes it possible to use transfer functions to produce accurate palaeonvironmental reconstructions. This type of work has been used extensively and very successfuly, particularly in palaeolimnology,and the
Environmental Change Research Centre at University College London
is at the forefront of such research. The Deep Sea Drilling Project (now the
Ocean Drilling Program
) has recovered many kilometers of cores and allowed the construction of a diatom biostratigraphy for most of the Cenozoic. Diatoms are particularly advantageous for biostratigraphic studies of high latitude sediments where calcareous microfossils are often poorly preserved, sparse, or of low diversity. |
 |
| Biology |
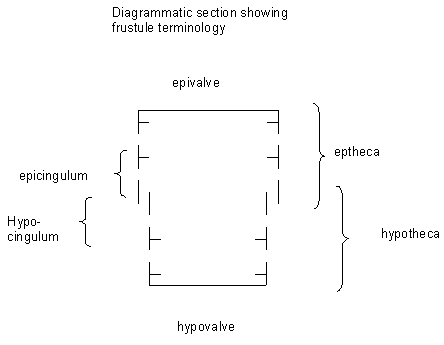
| Diatoms have been well studied both in their natural habitat and in cultures by biologists and there is therefore a wealth of knowledge on their biology and ecology. The protoplast of diatoms consists of a cytoplasmic layer that lines the interior of the frustule and surrounds a large central vacuole, within the cytoplasmic layer there is a diploid nucleus and two to several pigment-bearing plastids (the site of photosyntheseis). The diatom frustule is often likened to a pill-box or agar dish with an epitheca (larger upper valve), and a hypotheca (smaller lower valve). The vertical lip or rim of the epitheca is called the epicingulum, and the epicingulum fits over (slightly overlaps) the hypocingulum of the hypotheca. The epicingulum and hypocingulum with one or several connective bands make up the girdle. Many diatoms are heterovalvate, i.e., the two valves of the frustule are dissimilar. This is most obvious within the family Achnanthaceae where one valve has a raphe and the other does not, and the Cymatosiraceae where one valve has a tubular process and the other does not. Chain-forming species with cells linked together by siliceous structures may, in addition, have separation valves. These valves are morphologically different from the valves within the chain. Therefore, one species may have four morphologically distinct types of valves. |
 |
 |
| Life Cycle |
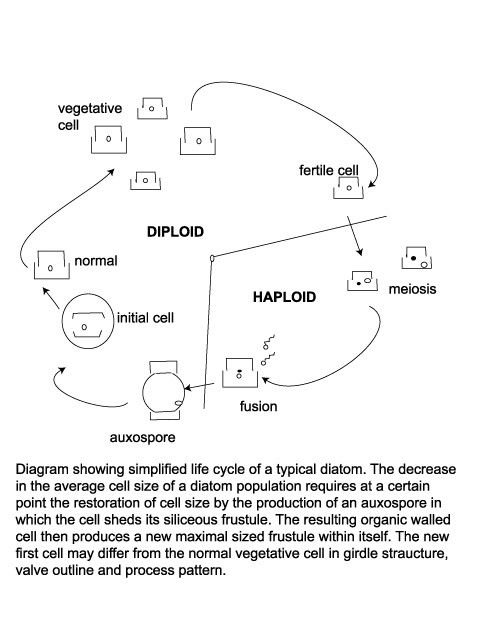
| When a cell divides each new cell takes as its epitheca a valve of the parent frustule, and within ten to twenty minutes builds its own hypotheca; this process may occur between one and eight times per day. Availability of dissolved silica limits the rate of vegetative reproduction, but also because this method progressively reduces the average size of the diatom frustule in a given population there is a certain threshold at which restoration of frustule size is neccesary. Auxospores are then produced, which are cells that posses a different wall structure lacking the siliceous frustule and swell to the maximum frustule size. The auxospore then forms an initial cell which froms a new frustule of maximum size within itself. Many neritic planktonic diatoms alternate between a vegetative reproductive phase and a thicker walled resting cyst or statospore stage. The siliceous resting spore commonly forms after a period of active vegetative reproduction when nutrient levels have been depleted. Statospores may remain entirely within the the parent cell, partially within the parent cell or be isolated from it. An increase in nutreint levels and/or length of daylight cause the statospore to germinate and return to its normal vegatative state. Seasonal upwelling is therefore a vital part of many diatoms life cycle as a provider of nutrients and as a transport mechanism which brings statospores or their vegetative products up into the photic zone. |
| The resting spore morphology of some species is similar to that of the corresponding vegetative cell, whereas in other species the resting spores and the vegetative cells differ strongly. The two valves of a resting spore may be similar or distinctly different. Often the first valve formed is more similar to the valves of the vegetative cells than the second valve. This diversity of the valve types belonging to the same species calls for caution in identification work using cleaned diatom material. |
 |
 |
| Preparation Techniques |
|
WARNING:
Please remember all preparation techniques require the use of hazardous materials and equipment and should only be carried out in properly equiped laboratories, wearing the correct safety clothing and under the supervision of qualified staff. |
| Diatoms are easily prepared for veiwing using a light microscope. Wet samples can be smeared onto a slide for immediate examination and determination of possible further treatments. Organic matter may obscure the detail of the frustule so this is commonly removed using hydrogen peroxide or some other oxidising agent. A small amount of hydrochloric acid may be added to remove any calcium carbonate and the sample then rinsed in distilled water until free of acids. The sample can then be dilluted and strewn onto coverslips, dried and mounted on slides. Because the refractive indices of water and silica are very similar, a mounting medium with a higher refractive index is used in order to increase the contrast. An excellent guide to preparing diatom slides is provided by the Geography Department at UCL. |
 |
| Observation Techniques |
| Diatom preparations may be observed using brightfield as well as phase contrast settings on a light microscope, the later is better for veiwing lightly silicified genera. Times forty dry or times one hundred oil-immersion objectives are most commonly used. The use of the scanning electron microscope allows the differentiation of processes if the inside of the valve can be veiwed. This is of particular use when attempting to speciate some of the centric diatoms. A guide to microscopy in relation to diatom preparations is provided by the Geography Department at UCL. |
 |
| Images |
| The following images are of a representative selection of diatoms aimed at giving a general overview of the different morphotypes. Each specimen is given a generic and if possible a species name followed by its age range, the site location from which the sample was obtained and its size in microns. Click on an image to view a larger version. Images are light microscope images unless otherwise stated. |

|
| Diploneis suborbicularis
(Gregory) Cleve |
|
|
| East Winch Borehole, Nar Valley, Norfolk, UK |
| 30 microns x 20 microns |

|
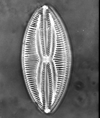
| Auliscus sculptus
(W. Smith) Ralfs ex. Pritchard |
|
|
| East Winch Borehole, Nar Valley, Norfolk, UK |
| 50 microns |
|
| Lyrella lyra
(Ehrenberg) |
|
|
| East Winch Borehole, Nar Valley, Norfolk, UK |
| 80 x 30 microns |

|
| Dimeregramma
sp. |
|
|
| East Winch Borehole, Nar Valley, Norfolk, UK |
| 50 microns |

|
| Nitzschia punctata
(W. Smith) Grun |
|
|
| East Winch Borehole, Nar Valley, Norfolk, UK |
| 45 x 15 microns |

|
| Cocconeis molesta var. crucifera
Grunow |
|
|
| East Winch Borehole, Nar Valley, Norfolk, UK |
| 30 x 15 microns (SEM) |

|
| Actinoptychus senarius
Ehrenberg |
| Cretaceous to Recent
|
| East Winch Borehole, Nar Valley, Norfolk, UK |
| 75 microns |

|
| Coscinodiscus radiatus
Ehrenberg emend. Sancetta |
| Eocene to Recent
|
| Chatham Deep, S.W. Pacific |
| 75 microns |

|
| Actinocyclus ingens
Rattray |
| Miocene to Pleistocene
|
| Louisville Moat, S.W. Pacific |
| 37 microns |

|
| Thalassiosira oestrupii
(Ostenfeld) Hasle emend. Fryxell and Hasle |
| Miocene to Recent
|
| Louisville Moat, S.W. Pacific |
| 10 microns |

|
| Thalassiosira lentiginosa
(Janisch) Fryxell |
| Mid to Upper Pliocene
|
| Chatham Deep, S.W. Pacific |
| 53 microns |

|
| Asteromphalus hookeri
Ehrenberg |
| Pliocene to Recent
|
| Bounty Fan, S.W. Pacific |
| 76 microns |

|
| Nitzschia reinholdii
Kanaya ex. Barron and Baldauf |
| Miocene to Pleistocene
|
| Louisville Moat, S.W. Pacific |
| 91 microns apical axis |

|
| Azpeitia tabularis
(Grunow) Fryxell and Sims |
| Miocene to Recent
|
| Chatham Deep, S.W. Pacific |
| 19 microns |

|
| Fragilariopsis ritscheri
Hustedt |
| Pliocene to Recent
|
| Louisville Moat, S.W. Pacific |
| 8 microns transapical axis (broken specimen) |

|
| Hemidiscus cuneiformis
Wallich |
| Miocene to Recent
|
| Chatham Deep, S.W. Pacific |
| 31 microns |

|
| Chaetoceros
Ehrenberg (Resting spore) |
|
|
| Chatham Deep, S.W. Pacific |
| 18 microns |

|
| Chaetoceros
Ehrenberg (Resting spore) |
|
|
| Louisville Moat, S.W. Pacific |
| 13 microns |

|
| Thalassiosira eccentrica
(Ehrenberg) Cleve |
| Recent
|
| North Chatham Terrace, S.W. Pacific |
| 25 microns |

|
| Cyclotella stelligera
(Cleve et Grunow) Van Heurck |
| Freshwater form
|
| North Chatham Terrace, S.W. Pacific |
| 27 microns |

|
| Stellarima microtrias
(Ehrenberg) Hasle and Sims (resting spore) |
| Recent
|
| North Chatham Terrace, S.W. Pacific |
| 50 microns |

|
| Thalassiosira ferelineata
Jouse |
| Recent
|
| Chatham Deep Terrace, S.W. Pacific |
| 30 microns |

|
| Psammodictyon panduriforme
(Gregory) Mann |
| Recent
|
| Chatham Deep, S.W. Pacific |
| 65 microns apical axis |

|
| Coscinodiscus radiatus
Ehrenberg emend. Sancetta |
| Eocene to Recent
|
| North Chatham Terrace, S.W. Pacific |
| 36 microns |

|
| Thalassiosira lineata
Jouse |
| Eocene to Recent
|
| Chatham Deep, S.W. Pacific |
| 30 microns |

|
| Thalassionema nitzschoides
(Grunow) Grunow ex. Hustedt |
| Miocene? to Recent
|
| Walvis Ridge, S.E. Atlantic |
| 72 microns apical axis |

|
| Stephanopyxis turris
(Greville and Arnott) Ralfs in Pritchard |
| Late Cretaceous to Recent
|
| Walvis Ridge, S.E. Atlantic |
| 23 microns (low focus) |

|
| Stephanopyxis turris
(Greville and Arnott) Ralfs |
| Late Cretaceous to Recent
|
| Walvis Ridge, S.E. Atlantic |
| 23 microns (high focus) |

|
| Azpeitia nodulifer
(Schmidt) Fryxell and Sims |
| Mid Miocene to Recent
|
| Walvis Ridge, S.E. Atlantic |
| 150 microns |

|
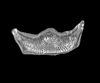
| Delphineis karstenii
Fryxell in Fryxell and Miller |
|
|
| Walvis Ridge, S.E. Atlantic |
| 32 microns |
|
| Eucampia antarctica
(Castracane) Mangin 1915 (girdle veiw) |
| Mid Miocene to Recent
|
| Walvis Ridge, S.E. Atlantic |
| 26 microns (horn to horn) |
 |