| Introduction |
| Acritarchs, the name coined by Evitt in 1963 which means "of uncertain origin", are an artificial group. The group includes any small (most are between 20-150 microns across), organic-walled microfossil which cannot be assigned to a natural group. They are characterised by varied sculpture, some being spiny and others smooth. They are believed to have algal affinities, probably the cysts of planktonic eukaryotic algae. They are valuable Proterozoic and Palaeozoic biostratigraphic and palaeoenvironmental tools. |
| Chitinozoa are large (50-2000 microns) flask-shaped palynomorphs which appear dark, almost opaque when viewed using a light microscope. They are important Palaeozoic microfossils as stratigraphic markers. |
 |
| History of Study |
| The earliest palynomorphs which we now classify as acritarchs are probably those reported by White in 1862 from the Ordovician to Devonian of New York. In the early 1930's Eisenack, working on material from the Baltic, re-assigned many species to the acritarch group and came to regard them (as we do now) as being derived from phytoplankton. It was Downie, Evitt and Serjeant however who tidied up the classification, providing a usable system, even though we are still uncertain of the groups true affinities. |
| Chitinozoa were first named by Eisenack in 1931, who noted the chitin-like wall material, (they are actually composed of a pseudochitinous material). As there are no living analogues we are again uncertain of their affinities, Kozlowski (1963) proposed they were the eggs of annelid worms. The co-occurrence of chitinozoa with scolecodonts and closely linked trend of abundance add weight to this proposal. In 1970 Jenkins recognised an affinity between chitinozoa and graptolites, again close associations of occurrence, geological range and trends of abundance were noted leading to his theory that graptolites and chitinozoa are probably genetically linked. Paris (1981) divided chitinozoa into two orders, the Prosomitifera and the Operculatifera. |
 |
| Range |
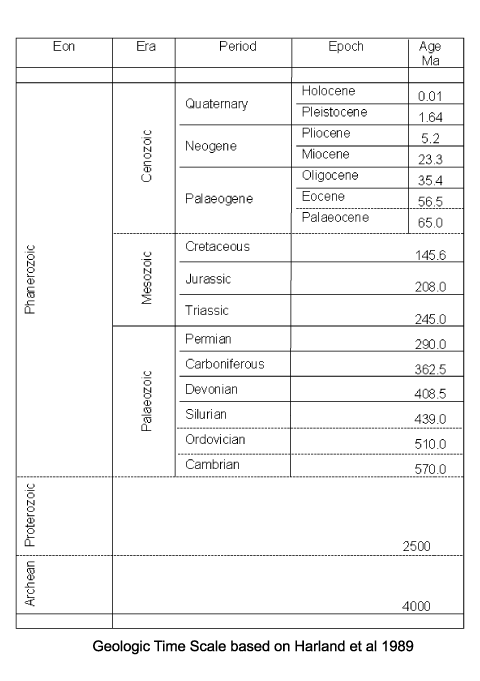
| The oldest known Acritarchs are recorded from shales of Palaeoproterozoic (1900-1600 Ma) age in the former Soviet Union. They are stratigraphically useful in the Upper Proterozoic through to the Permian. From Devonian times onwards the abundance of acritarchs appears to have declined, whether this is a reflection of their true abundance or the volume of scientific research is difficult to tell. |
| Chitinozoa first appear in the early Ordovician (Tremadoc stage) and became abundant and diverse by the Arenig and until the Silurian. Many genera became extinct at the end of the Silurian and few appear in the Devonian, they disappear in the earliest Carboniferous. |
 |
 |
| Classification |
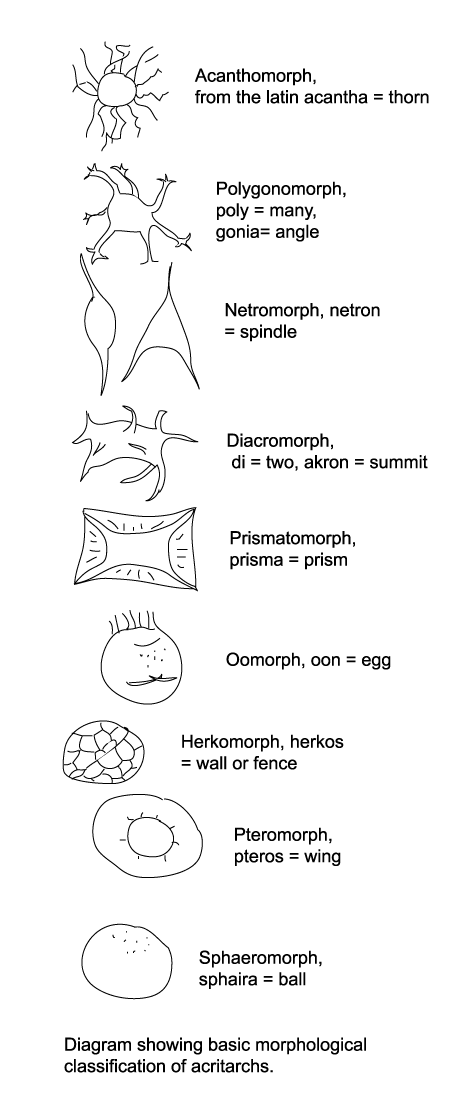
Natural classifications of acritarchs have not often been attempted nor accepted. The artificial scheme introduced by Downie, Evitt and Serjeant has been widely accepted and serves the purposes of most palynologists so there is little incentive to change it. Acritarchs are therefore divided into the following groups (most common first) based on their morphology.
- Acanthomorphs have spherical bodies with spines which usually open into the body.
- Polygonomorphs have a body-shape defined by the number and position of spines, they are often triangular or square in outline.
- Netromorphs have a fusiform body with one or more spines.
- Diacromorphs have spherical to ellipsoidal bodies with ornament confined to the poles.
- Prismatomorphs have prismatic to polygonal bodies the edges of which form a flange or crest which may be serrated.
- Oomorphs have an egg shaped body with ornament confined to one pole.
- Herkomorphs have a roughly spherical body divided into polygonal fields, rather like a football.
- Pteromorphs have a roughly spherical central zone often compressed, surrounded by a flange or wing lamella which may be sustained by radial folds or processes, they resemble under the light microscope a fried egg!
- Sphaeromorphs have simple spherical morphology.
|
 |
| Other groups once assigned to the acritarchs are now thought to be prasinophytes or other green algae, these include some species of herkomorphs, pteromorphs and sphaeromorphs. |
| Chitinozoa have been classified by Paris (1996) based on the structure of their openings and overall shape of flask and neck, again this is not a natural classification but it has become widely accepted. The Order Operculatifera containing one family the Desmochitina (simple spherical forms often found in chains) and the Order Prosomatifera containing two families the Conochitinidae (with little differentiation between flask and neck) and the Lagenochitinidae (with clearly distinct flask and neck). |
 |
| Applications |
| Acritarchs are extremely valuable for stratigraphic correlation of rocks of Proterozoic and Palaeozic age, primarily because they are the only microfossils commonly preserved. Chitinozoa are similarly utilised in studies of Palaeozoic strata. Both groups are also used in palaeoenvironmental reconstructions, providing useful evidence on environmental conditions where few other fossils exist. |
 |
| Biology |
| Acritarch morphology forms the basis of their classification therefore it is worth describing in some detail. Overall body shape ranges from spherical through fusiform (grain of rice shaped), stellate to prismatic. Processes may be simple or complex, solid or hollow, opening into the body or separated from it. Ornament may occur on the body and/or the processes. The form of opening called a pylome (through which excystment occurs) ranges from a distinctive opening in a defined area, a random split anywhere on the body or complete disintegration. |
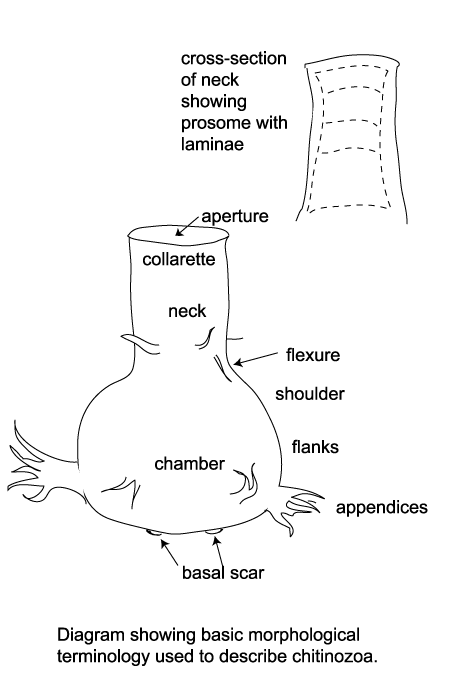 |
| Chitinozoa are flask-shaped sacs or vesicles, their surface may be smooth, striated, granular, spongy, spiny or ornamented with branching appendages. The main chamber may have a neck with a marked flexure between the two, the opening or pseudostome is covered by a disc or operculum which may be carried by a ringed stopper-like tube or prosome. The base of the vesicle may be convex, pointed or flat, sometimes with a crown of appendages. It is thought that at least some chitinozoa were linked together at these points to form colonial chains. |
 |
| Lifecycle |
| Acritarchs are extant but little is known about the organism which produces them. It is generally accepted that they are probably the resting cysts of marine phytoplankton and therefore represent only one stage of a multi-stage lifecycle. The gross morphology of acritarchs, a single organic sac or hollow vesicle, and their size, generally between 15 to 80 microns, suggests derivation from a unicellular organism. Many show simple excystment structures which may be divided into two groups: linear sutures which vary in position and shape, and pylomes which are complete small circular holes in the vesicle which may have a plug which fits in the hole called an operculum. These features are strongly suggestive of a dinoflagellate-like host organism. The fact that they are only found in rocks of marine origin, their great age and other preservational associations allows us to be almost sure they are wholly marine. |
| Chitinozoa are extinct marine organisms and it is therefore even harder to draw any inferences about their lifecycle from their shape and structure. They have been assigned to the protozoans, metazoans, protists and fungi, however some conclusions have been made. The chain-forming attribute of many chitinozoa is an important clue, the generally accepted theory is that the chitinozoa are the eggs of a metazoan, as long coiled chains of eggs are known in some groups for instance polychaete worms and molluscs. The fact that the chitinozoa vesicles of each species are all of the same size hints that they are the product of one life stage. There have been reports of "juvenile" chitinozoa but these have not been convincing and have not been generally accepted. |
 |
| Preparation Techniques |
|
WARNING:
Please remember all preparation techniques require the use of hazardous materials and equipment and should only be carried out in properly equipped laboratories, wearing the correct safety clothing and under the supervision of qualified staff. In particular the preparation of organic walled microfossils requires the use of potentially lethal chemicals and procedures and should only be carried out by trained personnel. |
| Clays, silts, mudstones, sandstones, limestones and shales may first be broken down physically into less than five millimeter fragments. The sample is then placed in a plastic beaker in a fume cupboard and covered in hydrochloric acid to remove carbonates. The sample is then removed from the hydrochloric acid and placed in hydrofluoric acid to remove silicates. Once removed from the hydrofluoric acid the sample is checked in a wet mount to ascertain the next procedure required. If the sample is clean of mineral matter it can be oxidised either using nitric acid or Schultze's solution. If mineral matter is present boiling in hydrochloric acid may be required before oxidation. The sample is removed from the last acid treatment and can either be swirled to separate organic matter from any remaining mineral matter or heavy liquid separation can be used where a heavy liquid with specific gravity of 1.6-2.5 is added to the sample and centrifuged, the organic material floating to the surface. |
| Two techniques for washing the acid out of the samples can be used. The decanting method involves allowing the acid and sample mix to settle and then carefully decanting off the acid, topping up with distilled water and repeating until the liquid is neutral. This is a time-consuming method. The second method is to wash the acid and sample mix through an acid resistant sieve gauze of a mesh size suitable to retain the palynomorphs. The mesh sizes often used are ten and twenty micron, ten micron gauzes being fine enough to catch all but the smallest spores and acritarchs, so if it is known these may be present five micron mesh size may be used. Chitinozoa are large enough to be captured in twenty micron gauzes and are often prepared for separately. |
| Staining of samples (with Safranin-0, a red colour, for example) may be used depending on the natural colour remaining in the palynomorph.
Coals and "coal-like" samples are often so devoid of mineral matter that the initial acid treatments can be omitted and oxidation only is required. This needs careful handling, however, as the oxidising reaction may be extremely vigorous. Kerogen slides are often required and require a special preparation technique without any oxidation or sieving to retain the total organic matter preserved in the sample. Once all acid treatment and separation processes are complete the sample may be mounted by strewing onto cover slips and allowing to dry. The inverted cover slip is then glued onto a slide using Norland Optical Adhesive or Petropoxy or similar proprietary glue of sufficient refractive index. |
 |
| Observation Techniques |
| Palynology slides are examined using transmitted light microscopes, commonly with times forty dry and times one hundred oil immersion objective lenses. Accurate co-ordinates of individual specimens on a slide may be required and are often given using either the graduated scale on the traversable slide table of a particular microscope or, preferably, by giving England Finder co-ordinates. An England Finder is a specially made slide which is divided into a grid of segments each one given an alpha-numeric reference. When a specimen is located on a slide the slide is carefully removed from the microscope and replaced by the England Finder and the co-ordinates marked down, as long as the orientation of the slide is also noted it is then possible to re-locate the specimen using the England Finder on any microscope. |
 |
| Images |
| The following images are of a representative selection of acritarchs and chitinozoa aimed at giving a general overview of the different morphotypes. Each specimen is given a generic and if possible a species name followed by its age range, the site location from which the sample was obtained and the magnification at which the image was taken or its size in microns. Click on an image to view a larger version. |

|
| Multiplicisphaeridium
sp.
|
| Silurian
|
| Rushbury, Wales |
| LM |

|
| colony of Leiosphaeridium sphaeromorph acritarchs
|
| Silurian
|
| Rushbury, Wales |
| LM |

|
| Crassiangulina
tesselita
|
| Silurian
|
| Rushbury, Wales |
|

|
| Neoveryhachium carminae
|
| Silurian
|
| Rose Hill, New York State, USA |
| LM |

|
| Fungochitina
sp. |
| Silurian
|
| Rose Hill, New York State, USA |
| length 250 microns LM |

|
| Fungochitinasp.
|
| Silurian
|
| Rose Hill, New York State, USA |
| length 250 microns LM |

|
| Fungochitinasp.
|
| Silurian
|
| Rose Hill, New York State, USA |
| length 250 microns LM |

|
| Lagenochitina sp. (right) Conochitina sp. (left)
|
| Silurian
|
| Rose Hill, New York State, USA |
|

|
| Lagenochitina
sp. |
| Silurian
|
| Rose Hill, New York State, USA |
| LM |

|
| chitinozoa
(aboral view) |
| Silurian
|
| unknown |
|

|
| Veryhachium
sp. |
| Silurian
|
| Rushbury, Wales |
| 19 microns excluding processes |

|
| Cymatiosphaera octoplana
|
| Silurian
|
| Rushbury, Wales |
| 25 microns |

|
| Leiofusa tumida
|
| Silurian
|
| Joslin Hill, New York State USA |
| 25 microns excluding processes |

|
| Veryhachium
cf. lairdi |
| Silurian
|
| Joslin Hill, New York State USA |
| 9 microns excluding processes |

|
| Veryhachium
cf. lairdi |
| Silurian
|
| Joslin Hill, New York State USA |
| 13 microns excluding processes |

|
| Multiplicisphaeridium
sp. |
| Silurian
|
| Joslin Hill, New York State USA |
| 20 microns excluding processes |

|
| Leiofusa
sp. |
| Silurian
|
| Joslin Hill, New York State USA |
| 7 microns excluding processes |

|
| Geron
gracilis |
| Silurian
|
| Joslin Hill, New York State USA |
| 12 microns excluding process |

|
| Geron
gracilis |
| Silurian
|
| Joslin Hill, New York State USA |
| 12 microns excluding process |

|
| Veryhachium limaciforme
|
| Silurian
|
| Joslin Hill, New York State USA |
| 25 microns excluding process |

|
| Multiplicisphaeridium
sp. |
| Silurian
|
| Joslin Hill, New York State USA |
| 20 microns excluding process |

|
| Veryhachium trispinosum
|
| Silurian
|
| Joslin Hill, New York State USA |
| 12 microns excluding process |

|
| Diexallophasis denticulata
|
| Silurian
|
| Joslin Hill, New York State USA |
| 12 microns excluding process |

|
| Veryhachium lairdi
|
| Silurian
|
| Joslin Hill, New York State USA |
| 12 microns excluding process |

|
| Multiplicisphaeridium fisherii
|
| Silurian
|
| Joslin Hill, New York State USA |
| 18 microns excluding process |

|
| Leiofusa bernesgae
|
| Silurian
|
| Joslin Hill, New York State USA |
| 10 microns excluding process |

|
| Geron
sp. |
| Silurian
|
| Joslin Hill, New York State USA |
| 21 microns |

|
| Veryhachium
sp. |
| Silurian
|
| Cyrenaica, Eastern, Libya |
| 40 microns excluding processes |

|
| ?Diacrodium?
sp. |
| Tremadocian (Lower Ordovician)
|
|
| 32 microns excluding processes |

|
| ?acritarch?
|
| Tremadocian (Lower Ordovician)
|
|
| 32 microns excluding processes |

|
| ?acritarch?
|
| Tremadocian (Lower Ordovician)
|
|
| 43 microns excluding processes |

|
| acritarch
|
| Tremadocian (Lower Ordovician)
|
|
| 45 microns excluding processes |

|
| acritarch
|
| Tremadocian (Lower Ordovician)
|
|
| 29 microns excluding processes |

|
| Priscotheca complanata
Deunff |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Shropshire, UK |
| 40 microns excluding processes |

|
| Stelliferidium cuvillieri
Deunff |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 38 microns |

|
| Cymatiogalea membranispinosa
Deunff |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 30 microns excluding processes |

|
| Vulcanisphaera brittanica
Rasul |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 30 microns excluding processes |
|
| Acanthodiacrodium angustum
Downie |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 36 microns |

|
| Vulcanisphaera imparila
Rasul |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 32 microns excluding processes |

|
| Vulcanisphaera africana
Deunff |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 48 microns excluding processes |

|
| Trichosphaeridium annovolaense
Timofeyev |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 46 microns |

|
| Stelliferidium cuvillieri
Deunff |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 32 microns |

|
| Cymatiogalea convexa
Rasul |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 31 microns |

|
| Priscotheca complanata
Deunff |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 33 microns |

|
| Saharidia fragile
Combaz |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 68 microns |

|
| Vulcanisphaera africana
Deunff |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 43 microns |

|
| Leiofusa squama
Deunff |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 80 microns |

|
| Archeohystrichosphaeridium funis
Rasul |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 32 microns |

|
| Leiofusa simplex
Rasul |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 45 microns |

|
| Impluviculus miloni
Vanguestaine |
| Tremadocian (Lower Ordovician)
|
| Tremadoc, Wales |
| 10 microns |
 |














































