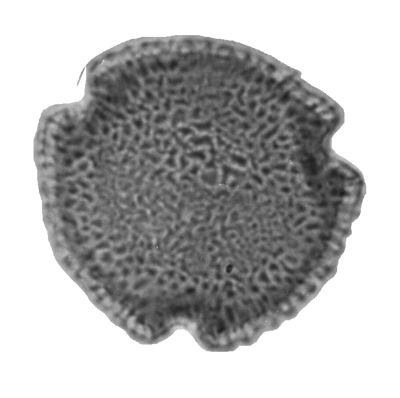
| Introduction |
| In general the spores of bacteria, fungi, algae and protists are rarely preserved but those of terrestrial plants are very common fossils. Terrestrial plants produce extremely resistent spores and pollen which are easily transported by wind and water. Most fossil spore and pollen grains are studied in a dispersed state and this is the fundamental basis upon which Hyde and Williams (1944) initially proposed the term Palynology. The initial meaning has now been expanded to include all acid-resistant organic microfossils. Spores are produced by the so-called "lower plants" or cryptogams, and within this group the pteridophytic vascular plants and bryophytes (mosses, liverworts and hornworts) are the most commonly studied. Pollen of seed plants, both angioperms and gymnosperms increasingly dominate palynological assemblages of Mesozoic and younger nonmarine deposits. |
 |
| History of Study |
| Observations of pollen by Grew and Malpighi are recorded from shortly after the invention of the microscope in the mid 17th Century. One of the very earliest practical applications of preserved pollen in the reconstruction of changing environments was by the Swedish palynologist Von Post in 1917. He studied tree pollen preserved in peat to build a picture of fluctuating climatic conditions during the Quaternary. In Britain during the 1930's Raistrick used spores he recovered from coals to recognise different coal seams, but he did not name the spores he found but assigned them an alpha-numeric code. Perhaps the greatest contribution made by a single person was that made by another Swede, Gunnar Erdtman, who from the 1950's to the 1970's produced several classic books and papers which remain required reading today. |
 |
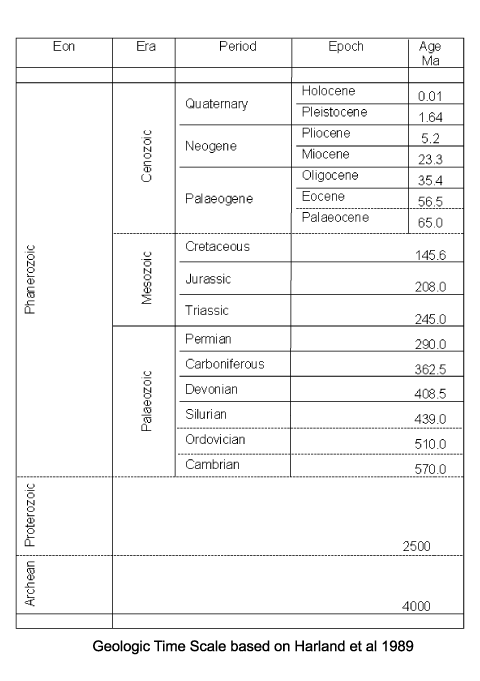
| Range |
| The earliest terrestrial plants are recorded from the late Silurian, and these were homosporous (all spores produced are of the same kind). By the end of the Devonian heterospory had appeared, this still involves dispersal by spores only but both microspores (held in a microsporangium) and megaspores (held in a megasorangium) are produced. Both these forms of plants relied on water (or at least damp conditions) to allow transport of the spermatozoid to the egg. The earliest gymnosperms appear in the very latest Devonian and rapidly become diverse and important during the Carboniferous. The angiosperms did not appear untill the early Cretaceous and diversified rapidly from the mid Cretaceous. |
 |
 |
| Classification |
| The fact that spores and pollen are normally retrieved from their host sediments as disjunct entities, separate from the original parent plant means that their natural affinities are often obscure. The free sporing plants including the Bryophyta e.g. mosses and liverworts, and the Pteridophytes which, although not a natural classification, encompassess all the seedless vascular plants, including the palaeontologicaly important ferns and fern allies, are primarily classified using the gross morphology, wall structure and the type of wall sculpture, if present. The important feature of homospory in terms of the fossil record is the four fold division involved in spore production, this takes the form of either a tetrahedra which gives a trilete spore or a tetragon which gives a monolete spore. The trilete and monolete marks imparted on the individual spores are the marks where each of the spore tetrad once abutted each other. |
| Classification of pollen, like that of spores is based on the morphological trends observed among various groups of fossils which may be primarily but not entirely reflections of evolution within the groups of plants which produced the pollen. It should also be remembered that higher plants have charcteristics of reproduction which permit them to utilise modes of evolution unavailable to animals. Because of their relatively simple genetic systems plants may utilise hybridisation and self fertilisation. The early gymnosperms produce prepollen, differentiated from true pollen by germination from the proximal rather than the distal side. Recent gymnosperms may produce very distinctive saccate pollen, i.e. pollen with one, two or rarely three air sacs attached to a central body (colpus) or monosulcate pollen as in the cycads and ginkgos. The angiosperms produce pollen with the greatest morphological variation, but typicaly with either a tricolpate or monosulcate form.(See Biology below). |
 |
| Applications |
| While botanical information from them may be limited, fossil spores and pollen have proved exceptionally useful as biostratigraphic indices. They are particularly valuable in freshwater environments, in evaporitic deposits and situations where marine and freshwater facies interdigitate. |
 |
| Biology |
| Spores, in the broadest sense, are produced in the life cycles of so called "lower plants" or cryptograms, comprising algae, fungi, bacteria and the extensive array of seedless metaphytes. Two basic forms of spore are recognised based on the original relationship of the spore tetrad when in the sporangium. In tetrahedral tetrads each of the four spores was in contact with all three of its neighbours on its proximal face, this gives each spore a distinctive trilete or Y-shaped mark. In the tetragonal tetrads each of the four spores was in contact with only two of its neighbours on its proximal face, this gives each spore a distinctive rectilinear scar and typically bean-shaped outline. An important point to remember when studying preserved spores is that they are generally compressed proximo-distally, that is along the polar axis, so care is required to differentiate betwen folds and trilete or monolete marks. Further subdivision of spores is based on wall sructure and ornamentation. |
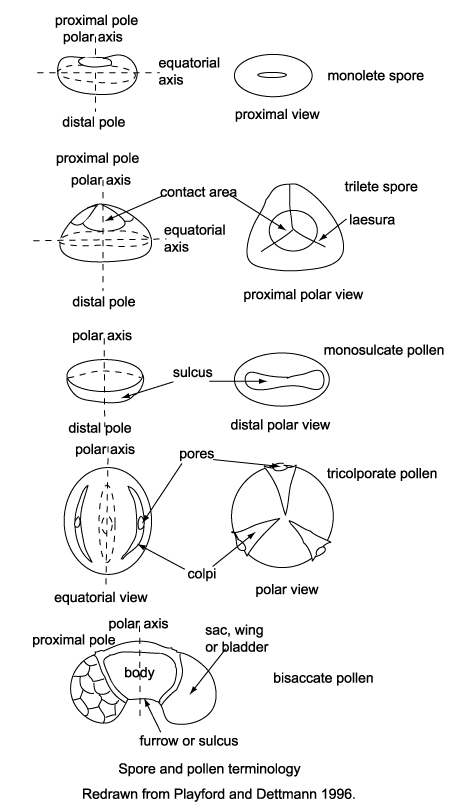 |
| Pollen is produced by seed plants, both angiosperms and gymnosperms. Pollen has a cellulose wall around the protoplasm called the intine, outside this is the sporopollenin layer which is inert, very tough and resistent to bacterial attack, this layer is called the exine. The pollen of several gymnosperm genera is saccate; that is, grains bear one, two or rarely three air sacs attached to a central body or corpus. Angiosperm pollen is extremely diverse and covers a multitude of combinations of features. Individual pollen grains may be inaperturate, or provided with one or more pores (monoporate, diporate, triporate, etc.), slit-like apertures or colpi (monosuclate, tricolpate, etc.), or a combination of pores and colpi (tricolporate, syncolporate etc.). |
 |
| Lifecycle |
| Vascular plants (those with special conducting tissues called xylem) have a similar alternation of generations as their algal forebears. Alternation occurs between spore-forming sporophyte generations (reproducing asexually) and a gamete-producing gametophyte generation (reproducing sexually with male and female gametes). In vascular plants, unlike algae, the sporophyte generation predominates, this is charcterised by the production at certain times of year of a spore-containing capsule called a sporangia. The first land plants to appear were homosporous, this means they produce spores of one kind only also called isospores. Modern ferns are homosporous, the diploid (2n) sporophyte plant produces by meiosis haploid (1n) spores in sporangia (sac-like packages) underneath the leaves. These are ejected onto the ground, which must be damp to allow the germination of the gametophyte (still haploid) stage. The gametophyte then produces either eggs or sperm, the spermatozoid swims to fertilise the egg, forming a diploid (2n) zygote. This then grows into the "adult" sporophyte ready to repeat the cycle. |
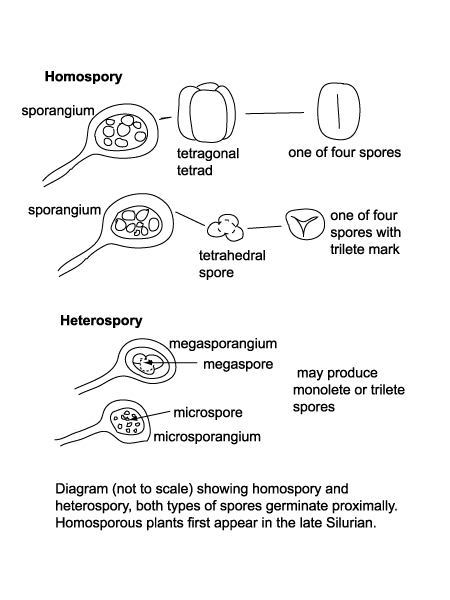 |
| The heterosporous life cycle differs from the homosporous in obvious terms by the production of differing spores, one type being large (megaspores) containing the eggs and the other being smaller (microspores) containing sperm. The eggs and sperm are produced within the spores whilst they are still on the parent plant. The spores are released from the sporangium and fertilisation takes place. This requires coincidental release, the advantage is that the megaspore contains a food reserve to give the new plant a head start, and only moist conditions are required for fertilisation to take place. In the fossil record megaspores are considered those spores over 200 microns across, which is a rather arbitrary division but is widely accepted. |
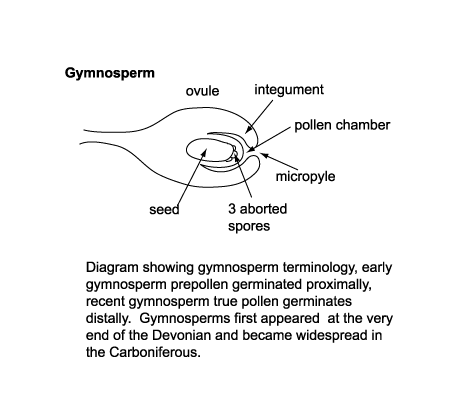 |
| In the seed gymnosperms (for instance pines) the megaspore is held within the plant and the microspore has to reach it by being carried by the wind, hence the very small size of gymnosperm "spores", which are called pollen. The pollen is trapped by a droplet of liquid at the entrance to a chamber which contains the megaspore. When pollen is trapped sperm travel down a special canal to fertilise the megaspore which remains within the plant and is fed by it. This produces a seed which contains its own food reserves ready to be released when conditions are right. The advantage over heterospory and homospory is that no water is required in the process but a vector (the wind) is needed and the process is very slow, for instance pines take two years to produce seeds after fertilisation. |
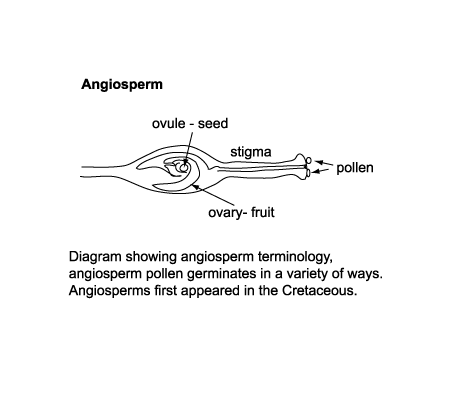 |
| The seed angiosperms differ in a very basic sense from gymnosperms in that they utilise a huge variety of vectors in addition to water or wind, such as butterflies, bees, and birds to carry the pollen to the megaspore. This requires a "reward" for the vector for instance nectar. Angiosperm pollen does not travel into the ovule but stays at the stigma (where it is deposited by the vector) and sends a nuclei to the ovule via a special tube. Once the egg has been fertilised it stays on the plant forming a seed surrounded by a fruit, which provides nurishment for the seeds inside. Because there is strong competition between pollen to fertilise the megaspore, angiosperms develop lots of apertures in the pollen so whichever orientation they are deposited on the stigma at least one aperture will be adjacent to the tube to the ovule. |
 |
| Preparation Techniques |
| WARNING:
Please remember all preparation techniques require the use of hazardous materials and equipment and should only be caried out in properly equiped laboratories, wearing the correct safety clothing and under the supervision of qualified staff. In particular the preparation of organic walled microfossils requires the use of potentially lethal chemicals and procedures and should only be carried out by trained personnel. |
| Clays, silts, mudstones, sandstones, limestones and shales may first be broken down physically into less than five millimeter fragments. The sample is then placed in a plastic beaker in a fume cupboard and covered in hydrochloric acid to remove carbonates. The sample is then removed from the hydrochloric acid and placed in hydrofluoric acid to remove silicates. Once removed from the hydrofluoric acid the sample is checked in a wet mount to ascertain the next procedure required. If the sample is clean of mineral matter it can be oxidised either using nitric acid or Schultze's solution. If mineral matter is present boiling in hydrochloric acid may be required before oxidation. The sample is removed from the last acid treatment and can either be swirled to separate organic matter from any remaining mineral matter or heavy liquid separation can be used where a heavy liquid with specific gravity of 1.6-2.5 is added to the sample and centrifuged, the organic material floating to the surface. |
| Two techniques for washing the acid out of the samples can be used. The decanting method involves allowing the acid and sample mix to settle and then carefully decanting off the acid, topping up with distilled water and repeating until the liquid is neutral, but this is a time-consuming method. The second method is to wash the acid and sample mix through an acid resistant sieve gauze of a mesh size suitable to retain the palynomorphs. The mesh sizes often used are ten and twenty micron, ten micron gauzes being fine enough to catch all but the smallest spores and acritarchs, so if it is known these may be present five micron mesh size may be used. |
| Staining of samples (with Safranin-0, a red colour, for example) may be used depending on the natural colour remaining in the palynomorph.
Coals and "coal-like" samples are often so devoid of mineral matter that the initial acid treatments can be omitted and oxidation only is required. This needs careful handling, however, as the oxidising reaction may be extremely vigorous. Kerogen slides are often required and require a special preparation technique, without any oxidation or sieving to retain the total organic matter preserved in the sample. Once all acid treatment and separation processes are complete the sample may be mounted by strewing onto cover slips and allowing to dry. The inverted cover slip is then glued onto a slide using Norland Optical Adhesive or Petropoxy or similar proprietary glue of sufficient refractive index. |
 |
| Observation Techniques |
| Palynology slides are examined using transmitted light microscopes commonly with times forty dry and times one hundred oil immersion objective lenses. Accurate co-ordinates of individual specimens on a slide may be required and are often given using either the graduated scale on the traverseable slide table of a particular microscope or preferably by giving England Finder co-ordinates. An England Finder is a specially made slide which is divided into a grid of segments each one given an alpha-numeric reference. When a specimen is located on a slide the slide is carefully removed from the microscope and replaced by the Englnd Finder and the co-ordinates marked down, as long as the orientation of the slide is also noted it is then possible to re-locate the specimen using the England Finder on any microscope. |
 |
| Images |
| The following images are of a representative selection of spores and pollen aimed at giving a general overview of the different morphotypes. Each specimen is given a generic and, if possible, a species name followed by its age range, the site location from which the sample was obtained and its size in microns. LM (Light Microscope) SEM (Scanning Electron Microscope). Typical and selected marker species are illustrated from each main period of the geological column in which spores and pollen occur. The images are divided into Cenozoic, Mesozoic and Palaeozoic forms, click on a link below or scroll down to each section. Click on an image to view a larger version. |
|
|
| Cenozoic |

|
| Tricolporopollenites
sp.
|
| ?Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 29 microns LM |

|
| Tiliaepollenites
sp.
|
| ?Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 22x20 microns LM |

|
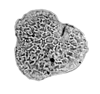
| Triporopollenites
sp.
|
| ?Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 25 microns LM |
|
| Retitricolporites
sp.
|
| ?Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 32 microns LM |

|
| Tricolporopollenites
sp.
|
| ?Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 35 microns LM |

|
| Tricolporopollenites
sp.
|
| ?Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 29 microns LM |

|
| Ongcosperma
sp.
|
| ?Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 25 microns LM |

|
| Perfotricolpites digitatus
(Gonzalez)
|
| ?Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 42 microns LM |

|
| Canthiumidites reticulatus
Khan
|
| Pliocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 31 microns LM |

|
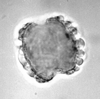
| Daemonorops sparsiflorus
Sowunmi
|
| Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 38 microns LM |
|
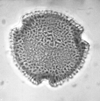
| Dicolpopollis
sp.
|
| Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 28 microns LM |
|
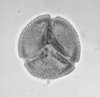
| Canthiumidites
sp.
|
| Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 40 microns LM |
|
| Myrtaceidites
sp.
|
| ?Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 28 microns LM |

|
| Monoporites
sp.
|
| ?Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 16x18 microns LM |

|
| Retimonocolpites
sp.
|
| ?Miocene?
|
| Kutai Basin, East Kalimantan, Indonesia |
| 24x34 microns LM |

|
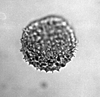
| Dracaena guienettii
|
| Neogene
|
| Pissouri, S.W. Cyprus |
| LM |
|
| Periporopollenites echinatus
(Wodehouse)
|
| Neogene
|
| Pissouri, S.W. Cyprus |
| LM |

|
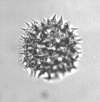
| Picaepollenites saccifer
Takahashi
|
| Miocene-Pliocene
|
| Pissouri, S.W. Cyprus |
| LM |
|
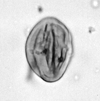
| Compositoipollenites rizophorus
Potonie
|
| Neogene
|
| Pissouri, S.W. Cyprus |
| LM |
|
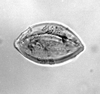
| Tricolporopollenites pseudocingulum
(Potonie)
|
| Lower Oligocene
|
| Pissouri, S.W. Cyprus |
| LM |
|
| Cycadopites (Monocolpopollenites zievelensis)
Pflug
|
| Upper Miocene
|
| Pissouri, S.W. Cyprus |
| LM |

|
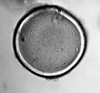
| Chenopodipollis multiplex
(Weyl and Pflug)
|
| Pliocene-Pleistocene
|
| Pissouri, S.W. Cyprus |
| LM |
|
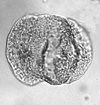
| Tasmanites
Newton (prasinophycean algal spore)
|
| Ordovician-Recent
|
| Pissouri, S.W. Cyprus |
| LM |
|
| Pinus haploxylon
Rudolph
|
| Miocene-Pliocene
|
| Pissouri, S.W. Cyprus |
| LM |

|
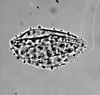
| Pinuspollenites taedaeformis
(Zaklinskaja)
|
| Middle Eocene-Miocene
|
| Pissouri, S.W. Cyprus |
| LM |
|
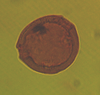
| Borassus flabellifer
|
| Middle Eocene
|
| Pissouri, S.W. Cyprus |
| LM |
|
| Pachydermites diederixii
Hopping and Muller
|
| Miocene
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |

|
| Echiperiporites estelae
Hopping and Muller
|
| Eocene-Miocene?
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |

|
| Crototricolpites crotonoisculptus
van Hoeken-Klinkenberg
|
| Palaeocene-Eocene
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |

|
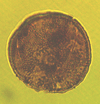
| Perfotricolpites digitatus
Gonzalez-Guzman
|
| Eocene-Miocene
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |
|
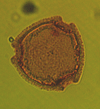
| Psilatricolpites crassus
van dr Hammen et Wymestra
|
| Eocene-Miocene?
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |
|
| Retitriporites
sp.
|
| Palaeogene?
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |

|
| Brevicolporites guinettii
Salad-Cheboldaeff
|
| Eocene-Miocene
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |
 |
| Mesozoic |
|
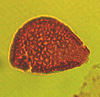
| Longapertites proxapertitoides
Van der Hammen and Garcia
|
| Maastrichtian (Upper Cretaceous)-Eocene
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |

|
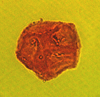
| Ephedripites barghoornii
Pocock
|
| Albian-Cenomanian (Cretaceous)
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |
|
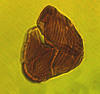
| Cicatricosisporites
sp. Potonie and Gelletich
|
| ?Upper Cretaceous?
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |
|
| Cretacaeiporites polygonalis
Herngreen
|
| Aptian-Cenomanian (Cretaceous)
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |

|
| Cretacaeiporites scabratus
Herngreen
|
| Cenomanian-Coniacian (Cretaceous)
|
| Keta-1 Well, offshore Accra-Keta Basin, Ghana |
| LM |
|
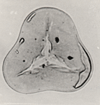
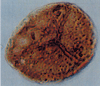
| Deltoidospora
sp.
|
| Turonian-Maastrichtian? (Cretaceous)
|
| Well C275-65, Sirte Basin, North Eastern Libya |
| 50 microns LM |
|
| Vallizonosporites pseudoalveolatus
Fensome
|
| Bajocian (Middle Jurassic)-Lower Cretaceous
|
| Outer Moray Firth Basin, North Sea |
| 54 microns LM |

|
| Lygodiosporites perverrucatus
Couper
|
| Middle Jurassic
|
| Outer Moray Firth Basin, North Sea |
| 90 microns LM |

|
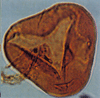
| Glaeicheniidites senonicus
Ross
|
| Middle Jurassic-Lower Cretaceous
|
| Outer Moray Firth Basin, North Sea |
| 30 microns LM |
|
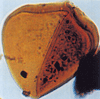
| Deltoidospora australis
Pocock
|
| Jurassic-Lower Cretaceous
|
| Outer Moray Firth Basin, North Sea |
| 70 microns LM |
|
| Dictyophyllidites harrisii
Couper
|
| Jurassic
|
| Outer Moray Firth Basin, North Sea |
| 44 microns LM |

|
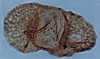
| Podocarpites reductus
Cookson
|
| Jurassic?
|
| Outer Moray Firth Basin, North Sea |
| LM |
|
| Alisporites thomasii
Nilsson
|
| Jurassic
|
| Outer Moray Firth Basin, North Sea |
| LM |
 |
| Palaeozoic |

|
| Dictyotriletes emsiensis
Mc Gregor
|
| Paraguian (Devonian)
|
| Teg and Reg fields, Timimoun Basin, West Algerian Sahara |
| 54 microns LM |

|
| Dibolisporites eifeliensis
Mc Gregor
|
| Paraguian-Emsian (Devonian)
|
| Teg and Reg fields, Timimoun Basin, West Algerian Sahara |
| 38 microns LM |
|
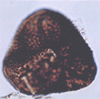
| Diatomozonotriletes franklinii
Mc Gregor and Camfield
|
| Emsian (Devonian)
|
| Teg and Reg fields, Timimoun Basin, West Algerian Sahara |
| 35 microns LM |

|
| Emphanisporites cf. pseudoerraticus
Schultz
|
| Emsian (Devonian)
|
| Teg and Reg fields, Timimoun Basin, West Algerian Sahara |
| 38 microns LM |

|
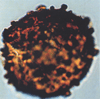
| Granulatisporites numinensis
Allen
|
| Emsian (Devonian)
|
| Teg and Reg fields, Timimoun Basin, West Algerian Sahara |
| LM |
|
| Raistrickia cf. clavata
Playford
|
| Tournaisian (Lower Carboniferous)
|
| Teg and Reg fields, Timimoun Basin, West Algerian Sahara |
| 38 microns LM |

|
| Auroraspora macra
Sullivan
|
| Tournaisian (Lower Carboniferous)
|
| Teg and Reg fields, Timimoun Basin, West Algerian Sahara |
| 45 microns LM |

|
| Dictyotriletes fimbriatus
Kaiser
|
| Tournaisian (Lower Carboniferous)
|
| Teg and Reg fields, Timimoun Basin, West Algerian Sahara |
| 45 microns LM |
 |