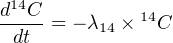
There are two stable isotopes of carbon: 12C and 13C, and one naturally occurring radionuclide: 14C. The half life of 14C is only 5,730 years, which is orders of magnitude shorter than the age of the Earth. Therefore, no primordial radiocarbon remains and all 14C is cosmogenic (see Section 8 for related methods). The main production mechanism is through secondary cosmic ray neutron reactions with 14N in the stratosphere: 714N (n,p) 614C. Any newly formed 14C rapidly mixes with the rest of the atmosphere creating a spatially uniform carbon composition, which is incorporated into plants and the animals that eat them. Prior to the industrial revolution, a gram of fresh organic carbon underwent 13.56 (β-) decays per minute. When a plant dies, it ceases to exchange carbon with the atmosphere and the 14C concentration decays with time according to Equation 2.2:
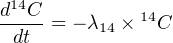 | (4.1) |
where λ14 = 0.120968 ka-1. Thus, the radiocarbon concentration is directly proportional to the radioactivity, which can be measured by β-counting. This can then be used to calculate the radiocarbon age by rearranging Equation 2.3:
![[ ]
t = --1-ln -d14C-∕dt--
λ14 (d14C∕dt)∘](index37x.png) | (4.2) |
where (d14C∕dt)∘ is the original level of β activity. This method was
developed by Willard Libby in 1949, for which he was awarded the Nobel
Prize in 1960. As mentioned before, (d14C∕dt)∘ was 13.56 prior to the
industrial revolution, when thousands of tonnes of ‘old’ carbon were
injected into the atmosphere, resulting in a gradual lowering of the
radiocarbon concentration until 1950, when nuclear testing produced an
opposite effect, leading to a doubling of the atmospheric 14C activity in
1963. Since the banning of atmospheric nuclear testing, radiocarbon
concentrations have steadily dropped until today, where they have almost
fallen back to their pre-industrial levels. But even prior to these
anthropogenic effects, 14C concentrations underwent relatively large
fluctuations as a result of secular variations of the Earth’s magnetic field
and, to a lesser extent, Solar activity. These variations in (d14C∕dt)∘ can be
corrected by comparison with a precisely calibrated production rate curve,
which was constructed by measuring the 14C activity of tree rings
(dendrochronology).
Since the 1980’s, β-counting has been largely replaced by accelerator mass spectrometry (AMS, see Section 3.1), in which the 14C concentration is measured directly relative to a stable isotope such as 13C. Although this has not significantly pushed back the age range of the radiocarbon method, it has nevertheless revolutionised the technique by reducing the sample size requirements by orders of magnitude. It is now possible to analyse individual seeds or tiny fragments of precious objects such as the Turin Shroud, which was dated at AD1260-1390.
Trace amounts of Rb and Sr are found in most minerals as substitutions for major elements with similar chemical properties. Rb is an alkali metal that forms single valent positive ions with an ionic radius of 1.48 Å, which is similar to K+ (1.33 Å). Rb is therefore frequently found in K-bearing minerals such as micas, K-feldspar and certain clay minerals. Strongly evolved alkalic rocks such as syenites, trachites and rhyolites often contain high Rb concentrations. Rb contains two isotopes of constant abundance: 85Rb (72.1854%) and 87Rb (27.8346%). Sr is an alkaline earth metal that forms bivalent positive ions with a radius of 1.13 Å, similar to Ca2+ (ionic radius 0.99 Å). It therefore substitutes Ca2+ in many minerals such as plagioclase, apatite, gypsum and calcite in sites with 8 neighbours, but not in pyroxene where Ca2+ has a coordination number of 6. Native Sr2+ can also substitute K+ in feldspars (where radiogenic Sr is expected to be found), but this substitution is limited and requires the simultaneous replacement of Si4+ by Al3+ in order to preserve electric neutrality. Sr therefore predominantly occurs in Ca-rich undifferentiated rocks such as basalts. Sr contains four isotopes (84Sr, 86Sr, 87Sr and 88Sr) with variable abundance due to the variable amount of radiogenic 87Sr. However, the non-radiogenic 84Sr/86Sr and 86Sr/88Sr-ratios are constant with values of 0.056584 and 0.1194, respectively. The Rb-Sr chronometer is based on the radioactive decay of 87Rb to 87Sr:
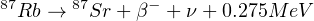 | (4.3) |
Where ν indicates an antineutrino. The number of radiogenic 87Sr atoms produced by this reaction after a time t is given by:
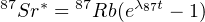 | (4.4) |
where 87Rb is the actual number of 87Rb atoms per unit weight and λ87 is the decay constant 1.42 × 10-11 a-1 (t1∕2 = 4.88×1010a). In addition to this radiogenic 87Sr, most samples will also contain some ‘ordinary’ Sr. The total number of 87Sr atoms measured is therefore given by:
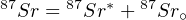 | (4.5) |
with 87Sr∘ the initial 87Sr present at the time of isotopic closure. Combining Equations 4.5 and 4.3, we obtain:
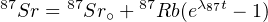 | (4.6) |
Dividing this by the non-radiogenic 86Sr yields
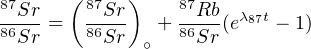 | (4.7) |
The method can be applied to single minerals or to whole rocks. Given the very long half life, the optimal time scale ranges from the formation of the solar system to the late Palaeozoic (300-400 Ma). To measure a Rb/Sr age, the weight percentage of Rb is measured by means of X-ray fluorescence, ICP-OES or similar techniques, and the 87Sr/86Sr ratio is determined by mass spectrometry (isotope dilution). The 87Rb/86Sr-ratio is then calculated as:
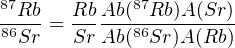 | (4.8) |
Where Ab(⋅) signifies ‘abundance’ and A(⋅) ‘atomic weight’.
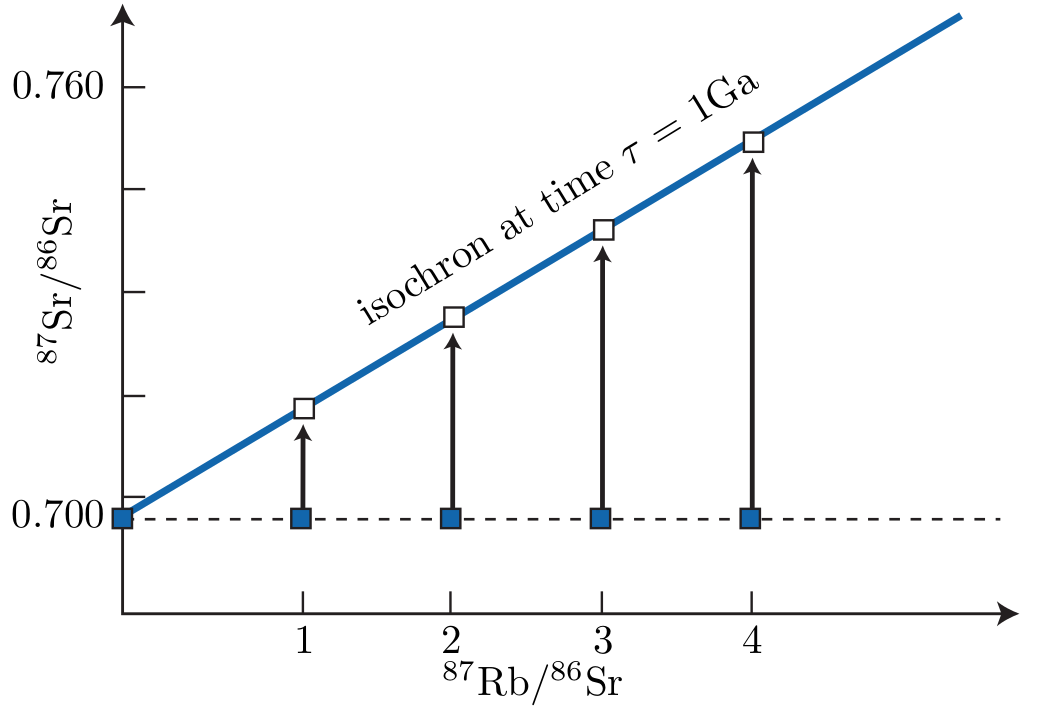
Equation 4.8 can be used in one of two ways. A first method is to use an assumed value for (87Sr∕86Sr)∘, based on the geological context of the sample. This method is only reliable for samples with a high Rb/Sr ratio (e.g., biotite) because in that case, a wrong value for (87Sr∕86Sr)∘ has only a minor effect on the age. A second and much better method is to analyse several minerals of the same sample and plot them on a (87Rb∕86Sr) vs. (87Sr∕86Sr) diagram (Figure 4.1). Due to Equation 4.7, this should form a linear array (the so-called isochron) with slope (eλ87t - 1) and intercept (87Sr∕86Sr)∘. Both parameters can be determined by linear regression, allowing us to quantify the ‘goodness of fit’ of the data and obviating the need to assume any initial Sr-ratios.
The elements Neodymium (Z=60) and Samarium (Z=62) are so-called ‘rare
earth elements’. All elements of this family have similar chemical properties.
They nearly all form 3+ ions of roughly equal albeit slightly decreasing size
with atomic number. The ionic radius of Nd and Sm is 1.08 and 1.04
Å, respectively. As the name suggests, rare earth elements rarely
form the major constituents of minerals. One notable exception is
monazite, which is a rare earth phosphate. In most cases, the rare
earth elements are found in trace amounts of up to 0.1% in apatite
[Ca5(PO4)3(OH,Cl,F)] and zircon [ZrSiO4]. Both Sm and Nd are
slightly enriched in feldspar, biotite and apatite and thus tend to be
found in higher concentrations in differentiated (alkalic) magmatic
rocks.
Because their chemical properties are so similar, geological processes are
rarely capable of fractionating the Sm and Nd concentrations. Therefore,
the Sm/Nd ratio in most rocks generally falls in a narrow range of
0.1 to 0.5 (the Sm/Nd ratio of the solar system being 0.31). One
exception is garnet, in which Sm/Nd ratios > 1 have been found. Partial
melting of mafic minerals such as pyroxene and olivine produces
lower Sm/Nd ratios in the fluid phase than the solid residue. The
Sm/Nd ratio of magmatic rocks therefore decreases with increasing
differentiation.
Natural Sm contains seven naturally occurring isotopes, three of which are radioactive (147Sm, 148Sm and 149Sm). Only 147Sm has a sufficiently short half life to be useful for geochronology. Nd also contains seven isotopes, of which only one is radioactive (144Nd) but with a very long half life. 143Nd is the radiogenic daughter of 147Sm and is formed by α-decay. This forms the basis of the Sm-Nd chronometer. Analogous to the Rb-Sr method (Equation 4.4), we can write:
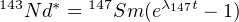 | (4.9) |
Hence:
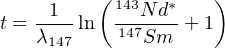 | (4.10) |
With λ147 = 6.54×10-12a-1 (t1∕2 = 1.06×1011a). Since most samples contain some initial Nd, the preferred way to calculate Sm/Nd ages is by analysing several minerals in a rock and create an isochron, similar to the Rb/Sr method (Section 4.3):
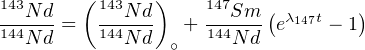 | (4.11) |
All measurements are done by mass spectrometry using isotope dilution.
Because of the identical atomic masses of 147Sm and 147Nd, it is
necessary to perform a chemical separation between Sm and Nd prior to
analysis.
The Sm/Nd method is generally applied to basic and ultrabasic igneous rocks (basalt, peridotite, komatiite) of Precambrian to Palaeozoic age. The method thus complements the Rb/Sr method, which is preferentially applied to acidic rock types. The Sm/Nd method can also be applied to high grade metamorphic rocks (granulites, eclogites) as well as meteorites (shergottites, nahklites). Since the rare earths are significantly less mobile than Rb and Sr, the Sm/Nd is more reliable in rocks that have been disturbed by weathering or metamorphism.