Updated Code of Conduct published

The new edition replaces the 2013 version, which was substantially revised to incorporate current expectations of researchers, and UCL commitments, as well as bringing together existing policies, processes, and external guidance that impacts upon research, and applying them into research practice. The Code is divided into three main areas:
Individual responsibilities
Your responsibilities throughout the research life cycle
Your responsibilities when reviewing, evaluating and editing other’s research
The Code was approved for publication in April 2023, following a period of consultation with the UCL community, and replaces all previous versions of the code. You can download the Code as a Pdf or accessible word version, or explore the dedicated webpages, where you can find additional information and guidance to help you understand how to apply the Code and were to find further help and support.
Thanks go to members of the Advisory Group and all those that responded to the UCL-wide consultation of the revisions. If you have any questions, please do not hesitate to contact the Research Integrity team via researchintegrity@ucl.ac.uk
Research Integrity e-learning course launched
UCL is committed to research integrity, and to supporting researchers to meet funder requirements relating to integrity related training. That’s why we have launched a self-paced eLearning course, Research Integrity, which provides a detailed explanation of what research integrity means for researchers at UCL and how it relates to your work.

Created by the Research Integrity team, the online training is available to all staff and students and consists of three modules:
- Introduction to research integrity – what is the purpose of research, how to ensure research benefits society, what research integrity is and why it matters, and UCL’s 3 E’s for research integrity (Every researcher, Every discipline, Everyday).
- Research integrity at UCL – outlines UCL’s approach to research integrity including the Statement of Research Integrity, the Code of Conduct for Research, and the Principles of Integrity.
- Leading by example - looks at what it means to lead by example, how to do it, and why it matters for research integrity and research culture at UCL.
You can access the course via UCL LearnUpon and through the Researcher Development catalogue throughout the academic year under the ‘Research Governance and Organisation’ section of the Researcher Development Framework (Introduction to research at UCL). Note: All PhD researchers are asked to access the course via inkPath in order to ensure your registration links to your training record.
On completion, learners are provided with a certificate which can be used as evidence of meeting learning requirements.
A further course ‘Research integrity in practice’ will be launched soon and will provide an in-depth look at how to embed the principles of integrity into each stage of the research lifecycle, as well as looking at cases studies and dilemmas.
If you have any questions about the course, please contact the Research Integrity team.
New Overseas Research Roadmap
Our updated guidance brings processes involved in planning and conducting international research together in one resource.
Doing research abroad or as part of an international collaborations is an exciting endeavor and can have many benefits. However, it also requires knowledge and understanding of different legal, financial and cultural requirements and scrupulous planning
We have updated the Overseas Research Roadmap to simplify finding the relevant information at UCL to help researchers comply with internal and external policies and regulations. The resource highlights the potential problems researchers might come across and points to where to find guidance and support.
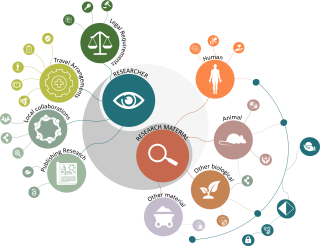
It is divided into two complementary parts:
- Researcher Information - things researchers need to consider for themselves when they intend to work abroad.
- Research Material Information - things to consider when working with specific sources of data or materials.
Let us know if you have any feedback or suggestions for improvement, please contact the Research Integrity team.
 Close
Close

