Trial of smartphone app to manage cirrhosis begins recruiting patients
27 November 2023
A smartphone enabled management system to enhance the care of patients with severe liver disease who are being discharged from hospital, is to be assessed in a trial led by UCL and the Royal Free Hospital, London.
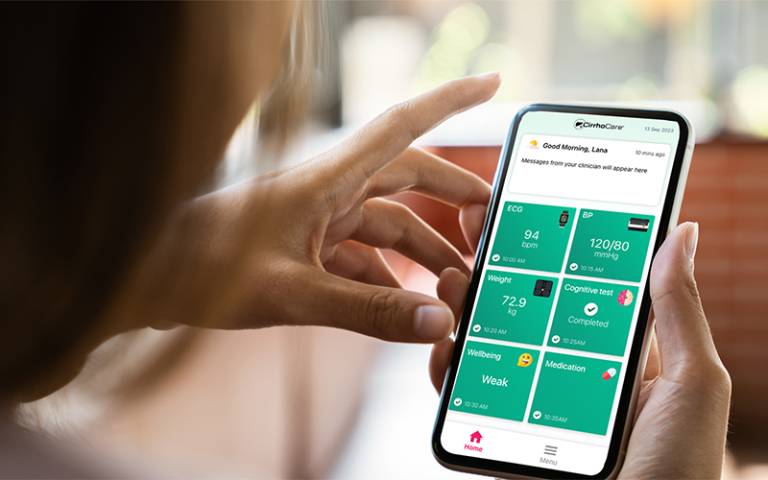
The system, called CirrhoCare, aims to improve care for people with advanced cirrhosis (scarring of the liver) who have recently been hospitalised – a group at high risk of severe ill health, of whom 37% are re-admitted to hospital within a month.
CirrhoCare includes a patient-facing app linked to smart devices designed to capture cirrhosis-related complications. It enables clinicians to monitor patients’ health remotely, allowing them to respond quickly if complications arise, with community-based interventions.
A pilot study in 2020, looking at 20 patients using the app alongside 20 controls receiving standard care, found that CirrhoCare reduced hospital readmissions by 38% over an average of 10 weeks, and substantially reduced hospital resource requirements, such as outpatient fluid drainage, by 80%. The findings were published in the Journal of Hepatology.
The new randomised controlled trial, which has received funding of around £2 million from the UK’s National Institute for Health and Care Research (NIHR), will be carried out in at least 12 NHS trusts and involves over 200 patients randomly allocated either to CirrhoCare or standard care
Professor Raj Mookerjee (UCL Division of Medicine and Royal Free Hospital, London), chief investigator of the trial, said: “Current care for advanced cirrhosis patients being discharged from hospital is limited to scheduled appointments every couple of weeks and is entirely reactive. People are identified as needing treatment only when they are very unwell and require emergency admission.
“This novel approach using CirrhoCare seeks to elicit signs of early deterioration so that patients needing treatment can receive prompt community-based intervention, so that further hospitalisations can ideally be prevented. In those with more severe deterioration, they can be prioritised for early hospital review, to offset emergency presentations.
“Deaths from liver disease have quadrupled since 1970 and are still increasing year on year. Liver disease is the third most common cause of premature death. However, there is significant regional variation in specialist liver services, with a disproportionate number of deaths occurring in the north of England.
“If CirrhoCare is shown to be effective, it will help ensure equal access to care. Whatever your postcode, you could have access to this platform and a specialist clinician, with intendant liver health and economic benefits.
“We have received all the required ethics and regulatory approvals and are now ready to open sites for patient recruitment.”
Before leaving hospital, patients on the CirrhoCare arm of the trial will receive a smartphone enabled with sim card, as well as a smartwatch enabling heart monitoring, digital blood pressure and temperature monitoring, and ‘smart’ scales which can capture weight and body fluid composition. They will also have access to an app to assess change in cognitive function in cirrhosis.
In addition, the kit will record sleep status and physical activity, and patients will answer questions when prompted via the app, for instance about how they feel or how much fluid they have drunk. The data is then analysed via algorithms which will flag to the clinical team if a complication may be occurring. A patient dashboard enables a quick, daily review by clinicians if prompted, and the app allows for two-way, secure communication between patient and clinician.
Pamela Everett, 71, from Holborn, whose cirrhosis is as a result of non-alcoholic fatty liver disease, took part in the initial trial. She said: “I only found out I had fatty liver disease after a routine blood test. I felt OK to begin with but progressively I felt worse and that’s when I was put on the trial. I have to say I found it really helpful, as it gave me peace of mind as I felt like I was being looked after 24/7. I did get a couple of phone calls during the trial because they noticed my blood pressure was quite low so we discussed that.
“The monitoring also reassured me I was getting plenty of sleep, which I was surprised about as I thought I wasn’t, and it also made me more mindful about what I was eating as my weight does go up and down a bit. To be honest I’d have happily carried on using it and I felt quite sad when it was time to give it back.”
The trial will assess the clinical effectiveness of the CirrhoCare system in reducing liver-related complications requiring hospital intervention, and patients in both arms will be followed up for 12 weeks following discharge from hospital. In addition, cost-effectiveness will be assessed, as well as impacts on patients’ quality of life and disease severity.
Ravi Kumar, founder and CEO of CyberLiver, said: “We thank NIHR for recognizing this significant unmet medical need, as well as the critical importance of providing innovative new digital intervention tools like CirrhoCare to patients with advanced cirrhosis. CirrhoCare is a UKCA Class IIa medical device and recognised by USFDA as a Breakthrough innovation, and this NIHR grant provides us with a remarkable opportunity to expedite the real-world adoption, and move forward with developing partnerships, that will help take CirrhoCare into clinical practice.
“The CyberLiver digital medicine platform provides a unique opportunity to use the power of digital therapeutics, artificial intelligence and sensor technologies to treat the increasing burden of liver disease. The expectation is that, as well as improving care for patients, it will be more cost-efficient, freeing up hospital resources, such as beds.”
The trial, led by researchers at UCL Division of Medicine, will be managed by the Comprehensive Clinical Trials Unit at UCL. It also involves researchers at the London School of Hygiene & Tropical Medicine.
Links
Image
- CirrhoCare app. Credit: CyberLiver.
Media contact
Dr Matt Midgley
E: m.midgley [at] ucl.ac.uk
 Close
Close

