The adult human retina contains a population of highly active metabolic cells, known as ‘Müller glia’. These cells have many important roles, including the provision of homeostatic and metabolic support to all retinal cells, and more importantly, they provide neuroprotection to retinal neurons by releasing a wide range of neuroprotective and antioxidant factors. In the adult zebrafish, there is evidence that these cells are responsible for the spontaneous regeneration of the retina after injury, yet, there is no evidence of their regenerative ability in humans.
Our group has shown that in the adult human eye, a population of Müller cells have the characteristics of stem cells. They can be isolated from the donor retina, and our laboratory has designed methods to isolate them from retinal organoids derived from embryonic stem cells (ES) or induced pluripotent stem cells (iPSC). In vitro, upon culture with growth and differentiation factors, they can be induced to differentiate into cells with the characteristics of retinal neurons.
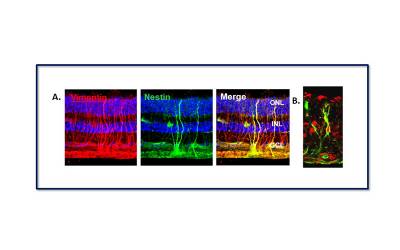 |
| Microscopic image of the human retina showing the presence of Müller glia with stem cell characteristics. (A) cells staining for the protein Vimentin (red), a marker of Müller cells, and Nestin (green), a marker of neural stem cells. Cells that stain for both proteins (yellow) are the Müller glial cells with stem cell properties. (B) Müller stem cell within the adult human retina staining for Nestin (green) and Sox2 (red). These two proteins are present in stem cells during foetal development. |
Due to the important metabolic functions that Müller glia have within the retina, as well as the stem cell properties that some of these cells exhibit, it is the aim of our lab to investigate their potential for retina regenerative therapies. Our main areas of research at present include the development of a Müller cell-based therapy for Glaucoma, investigations into the endogenous potential of Müller glia to regenerate the human retina, and examination of the neuroprotective ability of Müller released microvesicles.
1. Development of an adult stem cell-based therapy for Glaucoma
Glaucoma is a major cause of visual disability characterized by retinal ganglion cell (RGC) loss and damage to the optic nerve. Although current therapies can control disease progression, there is not available treatment for patients with late-stage or aggressive disease. Visual loss due to glaucoma is irreversible and blindness occurring with rapidly progressive disease may only be prevented by maintaining, repairing or replacing damaged RGC before total vision loss occurs. There is evidence that only a small number of retinal ganglion cells are needed to maintain visual function, for which providing neuroprotective and antioxidant factors to repair these cells in patients with late stages of disease could potentially maintain vision and improve their quality of life.
Our research has shown that intravitreal injection of Müller glia cells into the eye of animal models of glaucoma-like damage, causes improvement of retinal function. Although transplanted cells do not integrate into the retina or differentiate into neurons upon transplantation, they repair visual function in these animals, suggesting that they release neuroprotective factors to repair damaged RGC.
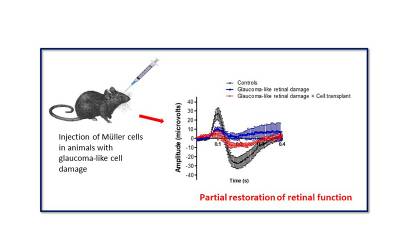 |
| Measurement of retinal function in animals with glaucoma-like damage following transplantation of Müller glial cells shows a significant improvement as judged by the scotopic negative responses to light (electro-retinography or ERG). Black line indicates normal ERG response, blue line indicates the response of animals with glaucoma-like damage and red line indicates the partially restoration of function by cell transplantation. |
Based on this data, we are currently supported by UCL Tech to develop a Müller cell therapy to prevent progression or improve RGC function in patients affected by glaucoma. The work involves the cooperation of non-clinical interdisciplinary scientists and specialist clinical investigators based at the UCL Institute of Ophthalmology (Cells for Sight Unit) and Moorfields Eye Hospital. To this aim, we have developed methods to produce Müller cells from retinal organoids under Good Manufacturing Practice (GMP), which we plan to test in humans upon safety validation.
2. Investigation of the endogenous potential of Müller glia to regenerate the human retina
Taking advantage of the potential that retinal organoids have in terms of harbouring different neural cell types and Müller glia, we are investigating whether Müller glia present within these organoids may be induced to proliferate and differentiate into photoreceptors in situ. Identification of molecules that may induce these cells to generate new neurons in vitro is being explored in our laboratory to potentially promote self-repair of the adult retina.
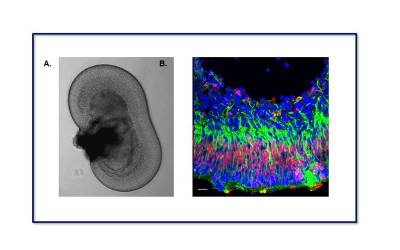 |
| Retinal organoids exhibit a characteristic anatomical structure that resembles the retina in vivo. (A) Phase-contrast micrograph showing a retinal organoid derived from embryonic stem cells in a tissue culture dish. (B) Confocal microscopy image of a retinal organoid section stained for Vimentin (green) and Chx10 (red), characteristic markers of Müller glia. |
3. Examination of the neuroprotective ability of Müller derived microvesicles
In addition to neuroprotective factors, secreted extracellular vesicles (EVs) are released by Müller glia. These nano-sized structures are known to contain protein and small non-coding RNA species capable of suppressing the translation of specific genes upon internalisation by other cells. It is therefore possible that EVs released by Müller glia may contain miRNAs with the potential to modify signal transduction pathways governing the growth and survival of neural cells, and this is the focus of our research. Our group are currently investigating the miRNA profiles of EVs produced by these cells and examining their potential for use in neuroprotective therapies to treat retinal degenerative diseases.
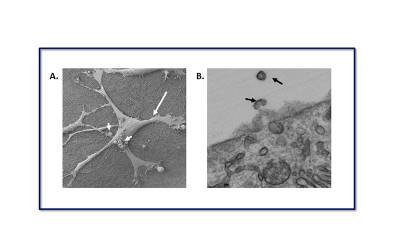 |
| (A). Scanning electron micrograph (SEM) shows a Müller glial cell in culture (large arrow). Microvesicles blebbing directly from the plasma membrane can be seen on the cell surface (short arrows). Scale bar: 50μm. (B). Transmission electron micrograph (TEM) shows a cross-section of an MIO-M1 cell. Black arrows show EVs being shed from the cell surface membrane. Scale bar: 500 nm |
 Close
Close

