Vascular Biology
Research into the role the vascular endothelium plays in the pathogenesis of brain and retinal disease is a core component of the laboratory.
Work from the Greenwood laboratory has been at the forefront of identifying and characterising novel endothelial cell signalling mechanisms that facilitate the recruitment of leucocytes to the brain and retina, a critical step in the pathogenesis of diseases such as multiple sclerosis, posterior uveitis and possibly diabetic retinopathy. Work from our laboratory has had a major influence on the decision to trial statin therapy for the treatment of inflammatory diseases of the brain and eye.
Our understanding of these cellular mechanisms remains superficial and ongoing research continues to identify other biochemical pathways that can equally be targeted through pharmacological intervention.
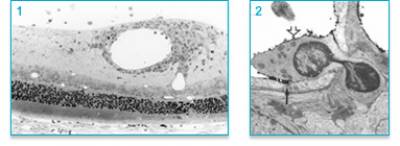
Figure 1: Perivascular leukocytic cuffing in rat uveitic retina. 2. Electron micrograph of mononuclear leukocyte penetrating the vascular wall of a retinal vessel.
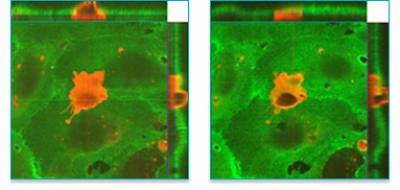
Figure 2: Lymphocyte migrating through junction of an in vitro brain endothelial cell monolayer.
Vascular angiogenesis/remodelling in the retina
In collaboration with the laboratory of Professor Steve Moss our other major focus is to identify novel drivers of vascular pathology in the brain and retina and in particular factors that contribute to the development of abnormal vessels.
This work has led to the identification of a number of secreted polypeptides that contribute to vascular remodelling including one that modifies TGFβ signalling. This work is currently leading to the development and testing of novel inhibitors that may prevent aberrant vascular development in diseases such as age-related macular degeneration (AMD) and diabetic retinopathy where new blood vessel growth is a major cause of vision loss.
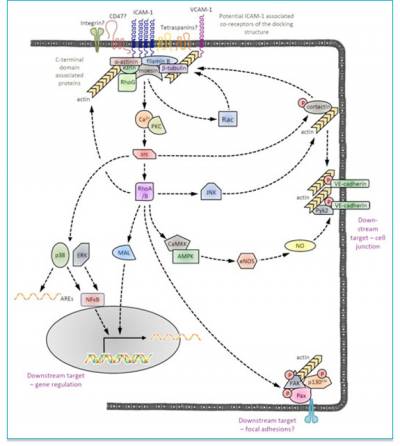
Figure 3: Schematic of identified/proposed ICAM-1 signalling pathways involved in facilitating leukocyte migration and modifying endothelial cell gene expression/function/.
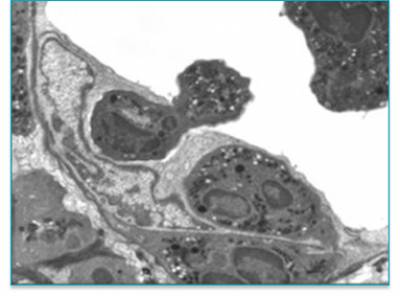
Figure 4: Electron micrograph of polymorphonuclear leukocytes penetrating retinal blood vessel wall (away from tight junctions) in an inflamed retina.
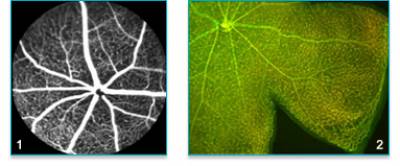
Figure 1: Fluorescein angiograph of normal mouse retinal vasculature. 2. Flat-mount of mouse retina with vasculature stained with isolectin B4 and claudin 5.
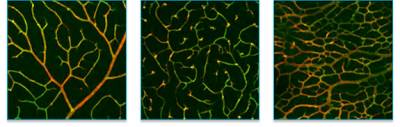
Figure 6: Superficial (left), intermediate (middle) and deep (right) vascular plexus of a P14 wild type mouse retina.
Retinal Pigment Epithelial (RPE) Cell Biology
RPE cells and AMD
Understanding the role of retinal pigment epithelial (RPE) cells in the development of retinal diseases constitutes another significant interest of the Greenwood laboratory.
In collaboration with Professor Moss we have been investigating the role of RPE in innate immunity and in the development of age-related macular degeneration (AMD). In particular we have investigated a cell therapy approach where we investigated the possibility of transplanting immortalised RPE to replace lost or dysfunctional host RPE.
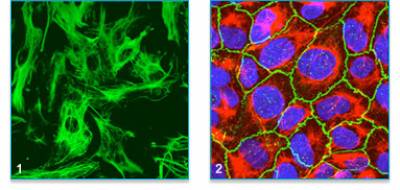
Figure 7:
1. Human RPE in culture stained with cytokeratin 8/18 antibody.
2. Cultured monolayer of human RPE stained for ZO-1 (green), cytokeratin 8/18 (red) and nucleus (blue).
More recently we have been studying the interplay between RPE cell function and the immune system and its potential role in the pathogenesis of AMD.
RPE cell transdifferentiation
Recently we have also been investigating the ability of RPE cells to transdifferentiate towards a neuronal phenotype. Whilst this work is in the early stages we have identified signalling pathways that appear to control this change in cell phenotype.
 Close
Close

