Study reveals novel method to deliver therapeutics to the brain
18 January 2022
The new method works by activating transcellular transport in vascular endothelial cells.
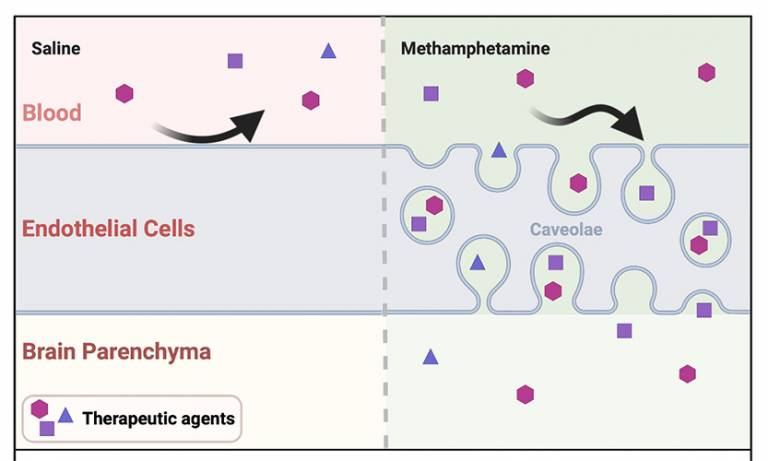
A new study titled "Methamphetamine enhances caveolar transport of therapeutic agents across the rodent blood-brain barrier" was published in Cell Reports Medicine this month. Led by IoO’s Dr Jui-Hsien Chang, in collaboration with the Department of Genetics at Trinity College Dublin, the researchers in Professor Patric Turowski's team asked whether non-specific vesicular transport could be stimulated at the blood-brain barrier and harnessed for the transport of chemical and biological agents to the rodent brain.
Entry of therapeutics to the brain is strongly limited by the blood-brain barrier, formed by the endothelial cells that line the blood vessels in the central nervous system (CNS). Blood-brain barrier endothelial cells are interconnected by highly impermeable junctions and also have largely suppressed non-specific transport through the cell via vesicles. To date, strategies to open the blood-brain barrier have focused on disrupting the intercellular junction complexes, thus opening a direct channel between blood and brain, or to piggyback therapeutics onto transport systems that provide the brain with essential nutrients. This latter approach is highly efficient for biological but not chemical agents and also requires elaborate and specific adaptation for each therapeutic.
The study found that in the vascular endothelium of the brain, low dose methamphetamine induces caveolar vesicles, which can efficiently transport both chemical and biological agents to the rodent brain. Using a model of Glioblastoma multiforme, low dose methamphetamine boosted the effectiveness of standard chemotherapy and significantly enhanced survival.
Methamphetamine is a psychoactive drug, which, at low doses, has FDA approval for medical use, e.g. for attention deficit disorders. The study shows that brief exposure to methamphetamine at blood concentrations presumed to be safe, opens the blood-brain barrier and allows blood-borne therapeutics to be accumulated more effectively in the brain. Future study will focus on identifying the cellular target of methamphetamine, so that the blood-brain barrier can be opened without any potentially undesired psychoactive side effects. In the meantime, methamphetamine may be trialed to make chemotherapy in glioma patients more effective. Additionally, methamphetamine will prove useful in facilitating and accelerating pre-clinical development of novel drugs for CNS diseases.
Professor Turoski and Dr Chang said:
“We are excited about this novel but simple strategy to transport therapeutics to the brain and hope it will considerably enhance existing treatments and speed up development of novel treatments for diseases affecting the brain.
Image
- Section of graphical abstract from paper
Links
- Research paper published in Cell Reports Medicine
- Link to open access article on Cell Reports Medicine
- Dr Cher Chang's academic profile
- Professor Turowski's academic profile
- Genetics Department at Trinity College Dublin
- Review of study on Bioworld
 Close
Close

