We hear from the researchers who are working to answer fundamental questions about the brain and advance the field through vital discovery research.
Investigating the role of brain immune cells in Alzheimer’s disease
Dr Soyon Hong, Group Leader at the UK Dementia Research Institute at UCL, is researching the role of microglia, the primary brain-resident immune cells, in Alzheimer’s disease. She tells us about her work.
What is your biggest motivation for pursuing your area of research?
I’ve always been motivated to understand how the brain works, so that we can understand how to fix it when things go wrong. What also motivates me is the hope that my lab’s efforts go towards something that can amount to change and give people hope. I started working on microglia as an undergrad—I thought they were the coolest cells (I still do!). In grad school, I wanted to learn how our brain degenerates and how we lose memory. So I joined the lab of Dennis Selkoe to investigate mechanisms of synaptic failure in Alzheimer’s disease. As a postdoc, I married these two interests and studied with Beth Stevens on the role of microglia in synaptic pathology.
What project is your main focus at the moment?
We aim to understand microglia better. We previously found that microglia, brain’s major immune cells, mediate the loss of synapses – the connection points between neurons – in mouse models of Alzheimer’s disease (Hong et al Science 2016). Microglia engulf, or eat, synapses; but we did not know what triggers this process. To that end, we recently uncovered a surprising role for SPP1, an immune protein secreted by neighbouring perivascular macrophages (immune cells that sit along the vasculature), in regulating microglial engulfment of synapses. Sebastiaan De Schepper, an absolutely brilliant postdoc in my lab, led this work, which was recently published in Nature Neuroscience. We’re now investigating which synapses are targeted by microglia and whether microglial eating of synapses is always a bad thing. We are also looking into the role of astrocytes, another type of specialised brain cell that sit right at synapses, in microglia-mediated synapse loss.
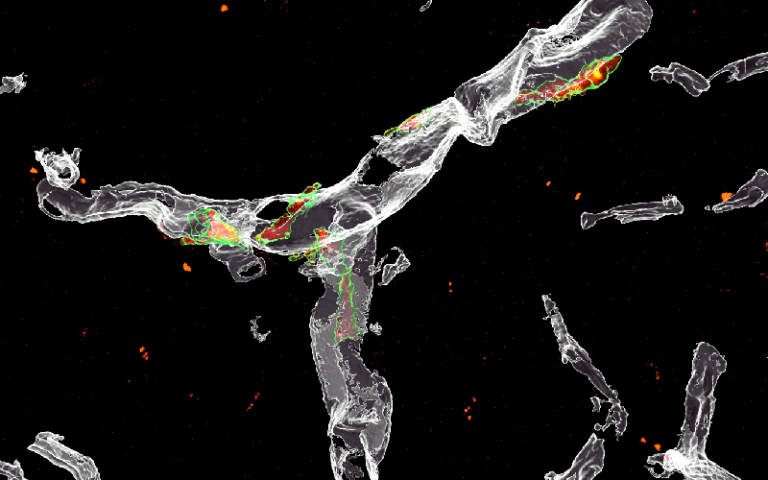
In a mouse model of Alzheimer’s, perivascular macrophages (green) along blood vessels (grey) in the hippocampus express SPP1 (red)/De Schepper et al., Nature Neuroscience.
What aspect of your work are you most excited about?
I’m excited about everything we’re doing! What’s important to us in the lab is to make fundamental discoveries –and hoping that in doing so, they will yield novel avenues to modify disease. Our brain is beautifully complex, and in the lab we are learning how different cell types in and outside the brain work together to keep the brain healthy and functional – the failure of such concerted effort can exacerbate disease. I think that is what makes what we do so exciting, biology is intricate and interdependent.
What have you found most challenging about your work so far?
I set up my lab four years ago. Less than a year after my first postdoc joined, the Covid-19 pandemic hit. We had to stop all experiments and deal with uncertainty around funding. It was a real test, especially for a young lab. I was worried also for my trainees’ present and future. But I was extremely fortunate because I was not alone in my struggle. I have a strong support network at home, and my lab is this amazing group of young people who are kind, resilient, and supportive, working interdependently as a team. I also have mentors whom I constantly tap into for advice.
How will your research impact people living with dementia?
Genetic studies in humans implicate microglia as risk modifiers for Alzheimer’s. Our research delves into what microglia do, especially their role in synapse loss, a pathological hallmark and a strong correlate of cognitive decline in patients. Now we have potential molecular avenues we can start testing to see if we can save synapses from aberrant elimination.
Who else is involved in your work and what is their role?
I have a team of really talented postdocs and students who drive their own projects that I think are fundamental to our understanding of the brain. To make and confirm a discovery takes collaborative efforts, which is one reason why I am grateful to be part of UCL and UK DRI. For example, because we work primarily with mouse models, it’s critical for us to think about the bigger picture and ensure that our work is relevant to human disease. When Christina Toomey (Queen Square Brain Bank) heard Sebastiaan’s talk on SPP1 at an internal seminar a couple years ago, she immediately reached out to Sebastiaan to collaborate. This led to a successful validation of our findings in mice in patient tissue with Tammaryn Lashley and her team. Also, when Michael Sasner at Jackson Labs sent us a new SPP1 mouse model he created, the wonderful Jemima Burden, who is the Head of Electron Microscopy at the UCL LMCB, performed ultrastructural resolution (CLEM) to confirm our findings. We were also lucky because our immediate neighbour was Carlo Sala Frigerio, a brilliant computational biologist whose expertise was critical to mapping SPP1 biology at single-cell level as well as cell-cell crosstalk.

What’s next for the Hong lab?
Following our finding that suggests SPP1 in regulating microglia-mediated synapse loss, we’re now working with Ionis Pharmaceuticals to see whether we can acutely target SPP1 to save synapses from loss in Alzheimer mouse models. We also found SPP1 as a key molecule involved in crosstalk between immune cells of the brain, so we are collaborating with Kenneth Harris and Bart De Strooper to map out such interactions using spatial transcriptomics. Finally, we’re looking at the role of SPP1 in amyloidosis, which is relevant because microglia that surround amyloid plaques express high levels of SPP1. So we’re investigating what happens to plaques and those plaque-associated microglia when you silence SPP1.
 Close
Close

