Using medical imaging biomarkers for healthcare research
2 January 2018
Learn how UCL researchers are using imaging biomarkers and innovative advanced imaging techniques to enhance diagnostic capabilities, monitor progress and advance understanding of disease.
Our exhibit at New Scientist Live will explore how imaging biomarkers can help detect and diagnose neurological conditions. In one of our demonstrations, we will be displaying MRI scans, clinical reports and 3D printed models of brains to show the difference that imaging biomarkers and quantitative analysis can make. There will be three scenarios shown; a control model of a healthy brain, a brain showing mild cognitive impairment and a brain with advanced Alzheimer’s Disease (AD). Attendees will be able to guess which brain aligns with which diagnosis and then learn more about why these correlate. Come and visit us at stall 1229 to try it out for yourself.
Imaging the brain for Alzheimer’s Disease research
Researchers within the Institute of Healthcare Engineering are experimenting with a variety of advanced imaging techniques to produce increasingly accurate images of the brain’s structure, assisting with the diagnosis and monitoring of neurological conditions.
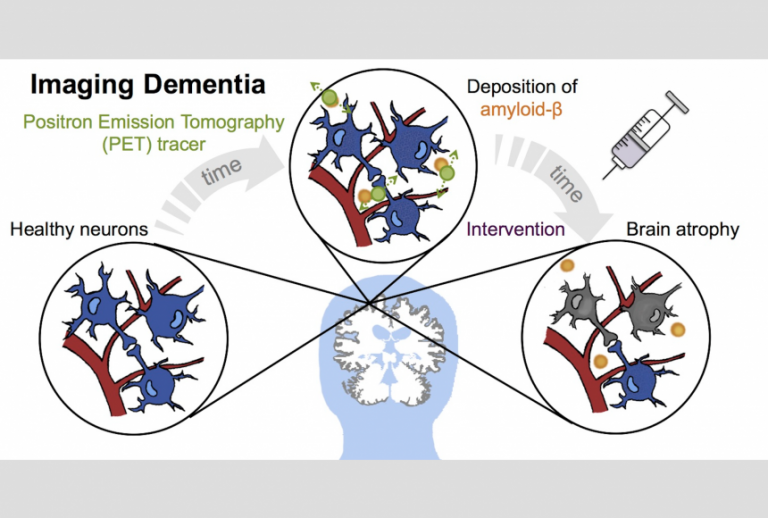
Enhanced imaging of the brain is essential to advance our understanding of AD and other neurodegenerative diseases. The potential patient impact of this is huge; AD affects more than 45 million people worldwide and there is a new case of dementia every 3.2 seconds. There is currently no cure for AD and treatment options are limited, however early diagnosis and intervention has been shown to have a significant impact on patients’ quality of life, slowing down the progression of disease.
This current prognosis means that the potential for research using simultaneous PET/MRI scanning to enable early detection of AD is extremely significant. Research has discovered that in the early stages of the disease lumps of sticky protein known as amyloid plaques are deposited in the brain, giving a good prediction of future brain atrophy. By using Positron Emission Tomography (PET) scans, which binds tracers to the amyloid proteins and allows them to be quantified, this can be detected before the patient has displayed any outward symptoms of cognitive impairment.

However, this method requires the patient to remain still in the scanner for 60 minutes or more which is not clinically feasible, particularly for patients with AD. To combat this, researchers at the Institute are focusing on reducing the acquisition time required to analyse the amyloid PET data. By using simultaneously acquired PET and magnetic resonance (MR) data, they are able to exploit the complementary information between the two modalities. Blood flow information from Arterial Spin Labelling (ASL) MRI is incorporated into PET kinetic modelling, reducing the amount of time needed to measure the amyloid proteins. This advanced technique could allow the detection of early indicators of AD to become a clinical reality. This work is carried out between the Translational Imaging Group (TIG), Institute of Nuclear Medicineand Dementia Research Centre. Read more here.
Other researchers are approaching AD from a different perspective and are looking at ways to link imaging data and genetics. This work is urgently needed due to the complex pathological nature of AD, which has meant that the disease’s heritability and genetic influences are still not well understood. Research is working to discover a susceptibility loci for AD which, to simplify, means a mutation in genes that could act as a predictor for future disease.

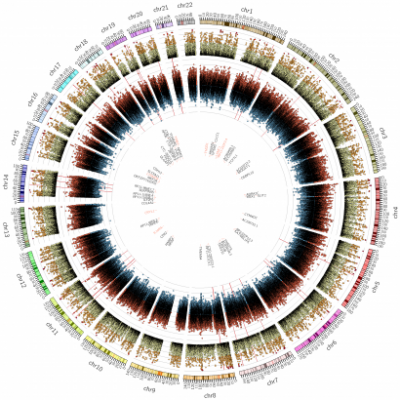
The uniqueness of the work taking place at the Institute of Healthcare Engineering is that rather than looking at the links between genetics and each pathological process of AD separately, it analyses them simultaneously to gain a more complete understanding. By combining this information researchers are able to gain a better measure of the disease’s overall severity and, using a mathematical model, are able to generate an individual score for each patient based on their stage of disease progression.
Using these scores, they performed a Genome Wide Association Study (GWAS) to look for genetic markers in the patients. The work found two new gene variations that correlate to imaging biomarkers of AD; one on chromosome 4 that is tightly linked to gene expression in the hippocampus and one on chromosome 22 that is related to amyloid accumulation. This has significant implications for future AD research and could, eventually, lead to novel therapies that target these chromosomes directly.
There are many other imaging techniques that are being used to advance neuroscience research within the Institute. For instance, dementia studies in collaboration with TIG and Dementia Research Centre have made use of resting state Functional Magnetic Resonance Imaging (fMRI) to show differences in the brain networks of different pathologies. Particular analysis of the cortical networks related to memory has revealed consistent network differences between a group of healthy controls and a group of individuals diagnosed with early-onset dementia. Again, these results show hope that very early intervention and diagnosis of AD could become a clinical reality for patients.
Advancing understanding of Multiple Sclerosis
Information gleaned from imaging the brain is not just used to enhance understanding of AD but to advance research into many other conditions. One example of this is the detection of biomarkers that indicate the progression of Multiple sclerosis (MS). In MS, the body’s immune system attacks and scars the protective covering (known as myelin) that surrounds the nerves of the central nervous system. The severity of these lesions along the brain and spinal cord can be used as biomarkers to monitor disease progression.
Using conventional imaging techniques, such as MRI, the lesions can resemble other types of pathology and can be difficult to identify. However, by combining imaging data with histology more information can be derived. 2D histological slices of an ex vivo brain allow researchers to investigate the brain’s precise microscopic structure. This information is then correlated with volumetric data from MRI scans to establish spatial correspondences. Taken together, this reconstructs a highly accurate atlas of the human brain. From this, researchers can derive a more realistic histological reconstruction and gain a more complete characterisation and understanding of MS as a disease. This work is done in collaboration with TIG and UCL Institute of Neurology.
Translational application of research?
A lot of the cutting-edge and experimental research in medical imaging going on at the Institute of Healthcare Engineering is at the earlier stages of its translational pathway. However, there are also examples of more developed research which has already reached the stage of trialling clinical applications and establishing spin-off companies.
BrainMiner is one example of this; it is an innovative imaging solution provider currently being trialled in prototype at University College Hospital. BrainMiner aims to enable quicker and more accurate diagnosis of AD and other forms of dementia. Conventional diagnosis occurs through analysis of MRI brain scans for signs of structural or volume change, which is time-consuming and dependent on a clinician’s qualitative assessment.
BrainMiner provides automated analysis of brain scans, enabling a more detailed and standardised approach. The software extracts robust, biologically meaningful measurements from clinical neuroimages, deriving accurate volumetric information about key brain structures. The company was established in 2015 out of the pioneering research and expertise in quantitative neurological imaging taking place within TIG. It is a collaboration between TIG, the National Hospital for Neurology and Neurosurgery (NHNN) and the Dementia Research Centre. Whilst BrainMiner is currently focused on advancing dementia research, it is hoped that this software could be applied more widely to other neurological conditions in the future, such as brain tumours.
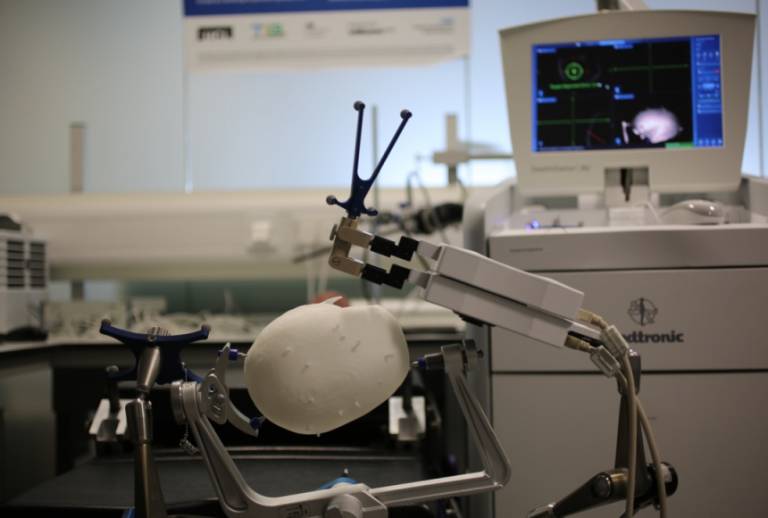
EpiNav™ is another example of the ongoing translation of research from within the Institute, with promising results from early clinical trials. EpiNav™ is an interactive neuro-navigation system used to assist in planning and guiding surgical interventions for patients with epilepsy (see image below). About 30-40% of epileptic patients continue to experience seizures despite optimum management of medication and are therefore potential candidates for curative resection, where the part of the brain responsible for emitting abnormal signals and causing seizures is removed. In order to identify the section to be removed, electrodes are implanted in an additional surgery before the resection procedure.
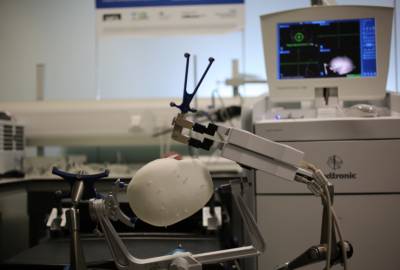

Whilst this has the potential to completely cure patients, this chance needs to be balanced by the risk that any inaccuracies during surgery could have huge implications, potentially causing new deficits such as paralysis or impaired speech and vision. EpiNav™’s software helps mitigate these risks by producing incredibly accurate maps of patients’ brain structures. This assists neurosurgeons pre-surgery, allowing them to plan a precise route for the electrode’s placement and trajectories that avoid the brain’s critical areas and blood vessels. EpiNav™ is a collaborative project between researchers from TIG, UCL Institute of Neurology and the NHNN.
The support and infrastructure provided by the Institute of Healthcare Engineering assists the transition of research into clinical application. The focus of all our research is getting patient-focused results and accelerating down the translational pathway to make an impact. To come and hear more about the advances we are making using medical imaging, please visit our team at stall 1229 at New Scientist Live.
New Scientist Live exhibition, London ExCel, 28 September 2017-01 October 201, Stand 1229.
 Close
Close

