Making the most of real world data
24 August 2017
 "> By Dr Nathan Davies from Primary Care and Population Health.
"> By Dr Nathan Davies from Primary Care and Population Health.
In this post Manuj Sharma talks about the mid-year meeting for the International Society of Pharmacoepidemioogy (ISPE) he recently attended at the Royal College of Physicians in London.
Pharmacoepidemiology itself comes across as quite a mouthful, but it simply refers to the study of the use and effects of medications in large numbers of people - focusing on both how effective and safe medications are. As such, research including both trials and observational studies focused on medication all come under the pharmacepidemiological heading.
A big point for discussion at this years conference was the impact of ever growing volumes of "real world" patient data and what it means for the field of pharmacoepidemiology going forward. "Real world" is any data collected outside of the constraints of conventional randomised trials. When it comes to medications, there are many who traditionally have looked at randomised controlled trials as the only means of getting to a clear unbiased answer but given so much data is now becoming accessible, can we really afford to ignore other study designs and methods?
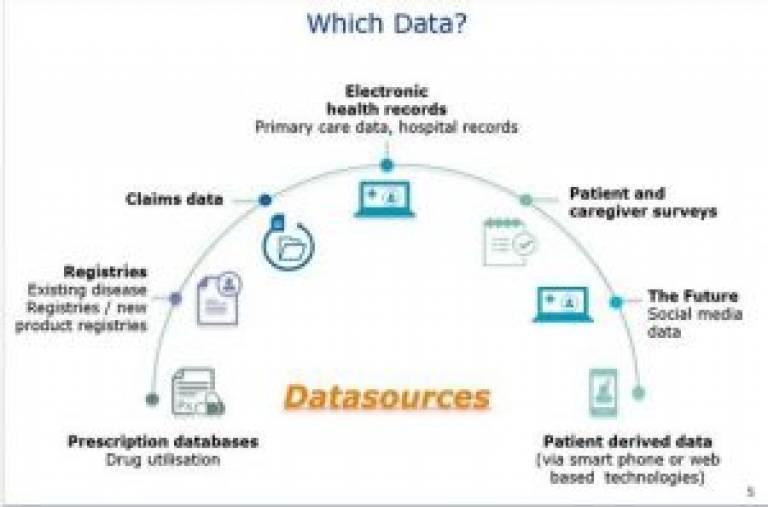
"Real World" Data Sources. Reproduced with permission from presentation delivered by Dr Enrica Alteri from European Medicines Agency at the ISPE Mid-Year Meeting in London, 2017
The thoughts on what all this new patient data meant for the future of research into medication were sought from representatives from 3 key stakeholders, regulators, industry, and the NHS. The discussion was extensive but here are some of the major points that grasped my attention and gave, I felt, most food for thought…
The regulators were up first and their perspective was delivered by Dr Enrica Alteri, Head of Research and Development at the European Medicines Agency (EMA). She was quick to emphasise how the EMA have been advocating increasing use of "real world" data for some time.
The most interesting example provided of this was regarding an extension of licensing granted to a medication called Soliris® (eculizumab), used for paroxysomal nocturnal haemoglobinuria, a life threatening condition where red blood cells break apart prematurely. The original trials approved the medicine for use in particular patient group with history of blood transfusions while a registry based study using "real world data" was subsequently used to successfully extend the license of the medication for use in patients with other levels of disease severity. The path that any new treatment takes from development to decisions on approval and reimbursement can take over 20 years. So with new medicines being developed at a faster pace, there is a need to make this process more efficient to allow patients to safely access treatments sooner. The EMA is accepting of this type of licensing extension using "real world" data, provided of course, like with any trial, the study is conducted in a rigorous, robust manner!
The industry perspective came from Andrew Roddan, Vice President & Global Head of Epidemiology at GlaxoSmithKline who highlighted another important role for this data in drug development through identifying disease patterns and targets. He also emphasised that use of "real world" data and undertaking randomised trials did not have to be mutually exclusive. Andrew Roddan used The Salford Lung Study as an exciting example where professionals from eight organisations across Greater Manchester involving over 2,800 patients, 80 GP practices and 130 pharmacies collaborated to investigate effectiveness of a new inhaler, Relvar Ellipta® for COPD. The study was securely hosted within the NHS network, which integrated the electronic medical records of consenting patients across all of their everyday interactions with their GPs, pharmacists and hospitals. This linked database system allowed monitoring of patients' safety in close to real-time with minimal intrusion into their daily lives. This also meant recruitment of a large group of patients was possible - including types often excluded in traditional respiratory trials. Not everywhere has the technological integration that is in place in Salford, but this was an exciting vision for where medicines development can go!
The final perspective, fittingly came from Dr Indra Joshi who gave her viewpoint from the NHS frontline, as a practising acute medicine physician. Dr Indra Joshi was excited by the increasing volumes of patient data emerging and believed it could greatly contribute to various aspects of pharmacoepidemiology while also improving patient care. She was, however, keen to remind everyone that patients must be involved each step of the way to ensure this data continues to become available. She also believes that despite advances there was still some way to go before we achieve the healthcare record integration needed to conduct studies to the quality of the Salford Lung Study throughout the UK.
The lively discussion and presentations from the stakeholders gave interesting perspectives on the future of growing volumes of patient data and what it meant for research into medication. Despite some differences there was overall agreement on a few points. While trials remain central to medication licensing decisions and establishing efficacy of treatments early on, there are multiple opportunities for "real world" data to add to this evidence base to support the process. A clear understanding of the strengths and limitations of the available "real world" data was key to realising where they can add most value. And finally, how important it is to ensure early and frequent engagement between all stakeholders for success. Next time, it would be great to hear the patient perspective as well!
 Close
Close



