In medical research there are two main types of studies:
- Observational Studies – These studies do not involve a new intervention or treatment option. They aim to find out information by observing the people taking part and see what happens in different situations.
- Interventional Trials – These aim to find out information about a particular intervention or treatment. Intervention Trials, called Clinical Trials, are the gold standard for finding out if a new intervention or treatment option(s) is suitable for people.
Clinical Trials:
Researchers will run clinical trials in stages. The early phases aim to find out more about the safety and side effects of a new treatments. Later phases aim to see if a new treatment works better than the current treatment.
DIFFERENT STAGES OF A CLINICAL TRIAL
- Phase I - Trials involves a small number of people (~20-50 people) and looks at what happens to the treatment in the body and if there are any side effects. The trial also looks to find the best dose for the treatment.
- Phase II - Trials involve a medium number of people (~over 100 people) and looks at how well a treatment works.
- Phase III - Trials involve many people (~100s or 1000s of people) at compares the new treatment against the standard treatment option to check if the new treatment is better and/or has fewer side effects.
- Phase IV - These trials involve 100s or 1000s of people depending on the investigation and look at finding out more about the long-term benefits and side effects of the new treatment.
Here we described a few different trial designs which you may come across in your visit to the Clinical Research Facility:
First-In-Human (FIH) Studies:
First-in-human (FIH) studies are a type of clinical trial in which a new drug, procedure, or treatment is tested in humans for the first time.
First-in-human studies take place after the new treatment has been tested in laboratory and animal studies and are usually done as Phase I clinical trials.
Dose Escalation Studies:
These trials can sometimes to be referred to as Phase I Dose-Finding Studies.
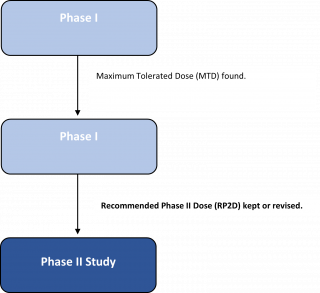
A study that determines the best dose of a new drug or treatment. In a dose-escalation study, the dose of the test drug is increased a little at a time in different groups of people until the highest dose that does not cause harmful side effects is found - called the Maximum Tolerated Dose (MTD).
The trial will aim to find the MTD which does not exceed a pre-set limit of side-effects - called the Dose Limiting Toxicities (DLT).
Together with these two points of data, the trial can find the Recommended Phase II Dose (RP2D). If successful, this will be the dose which can be used in Phase II.
A dose-escalation study may also measure ways that the drug is used by the body.
This design of trial is usually part of a Phase I clinical trial.
Dose Expansion Studies:
These trials are sometimes done to compliment Dose Escalation Trials by further investigating to find the Recommended Phase II Dose (RP2D).
These trials may have stricter criteria and may look at disease-specific cohorts. They are usually done in the same Phase I trial as Dose Escalation Studies.
Master Protocol Trials
A Master Protocol Clinical Trial includes multiple subgroups and sub studies, with patients having same or different diseases and that employ one or multiple interventions/drugs to treat it.
This type of Protocol design has been developed for, and is common across, Oncology, in which one investigational drug/treatment and/or more than one cancer type can be investigated within the same trial structure.
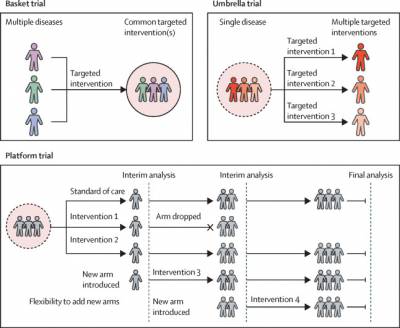
There are three types of Master Protocol Designs: Basket Trials, Umbrella Trials and Platform Trials.
Basket Trials:
Also referred to as Multiple Cohort Clinical Trials.
In this trial design patients from different disease groups or subgroups, such as those with different types of cancer, are identified within those groups based on the presence of a specific factor.
Basket designs are intended to study a single investigational regimen in a number of different diseases or disease subtypes.
A basket trial enrols patients with different cancer types and/or different cancer subtypes but who share a common link, usually having the same genetic or molecular abnormality.
For example, the trial would test a new intervention or treatment in a select number of different cancer types (e.g., Colon, Breast, Lung, and others) and/or different cancer subtypes (e.g., Lymphoma Cancers - Hodgkin Lymphoma, Non-Hodgkin Lymphoma, Chronic lymphocytic Leukaemia (CLL), and others).
Patients with that specific ‘common factor’ who take part in Basket Trials are grouped together in one cohort, or “basket,” to receive a new intervention/treatment which is designed to work, or to work best, in that specific category of disease.
Umbrella Trials:
Umbrella trials, on the other hand, evaluate multiple different targeted interventions/treatments for a single cancer type or sub-type that is stratified into subgroups by molecular alternation.
An umbrella trial enrols patients with one cancer type but with different genetic changes within each tumour. It consists of many small sub-trials to test multiple drugs simultaneously in one large trial.
Patients receive different targeting treatments matched to their genetic aberration. The term “umbrella” refers to separation of one alleged cancer into many sub-cancers depending on their molecular features. There is also a “default arm” which assigns patients without a specific marker to receive standard treatment.
Basket trials and umbrella trials both employ a molecular screening protocol that allows either recruitment of different diseases with the common molecular alteration(s) or that differentiates the single disease into different molecular subtypes.
Platform Trials:
Also referred to as Multi-Arm, Multi-Stage (MAMS) design trials.
A multi arm trial is a trial that has:
- several treatment groups as well as
- the standard treatment group (the control group Open a glossary item)
Multi-arm multi-stage (MAMS) trials have the same control group all the way through. The other treatment groups can change as the trial goes on.
The research team may decide to stop recruiting people to a particular group. This could be because they have enough people to start looking at the results. Or because early results show the treatment isn’t working as well as they’d hoped.
The researchers may add new treatment groups as new drugs become available to look at. This means they don’t have to design and launch a brand-new trial each time they want to research a new treatment. So, it helps get results quicker.
 Close
Close

